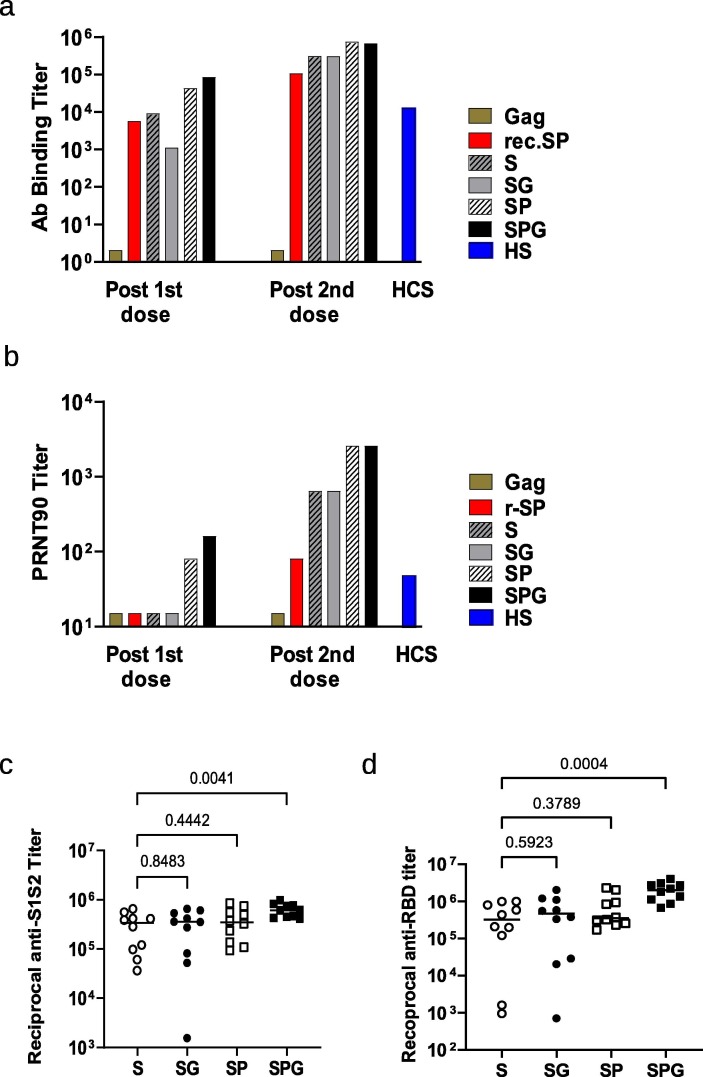
Fig. 2.
Immunogenicity of the various forms of SARS-CoV-2 S eVLPs in C57BL/6 mice. C57BL/6 mice, 10 per group, received 2 injections of Gag eVLPs deprived of S or various forms of SARS-CoV-2 S or recombinant prefusion SP at day 0 and 21 as indicated on legend, S: native S, SG: S with VSV-G tail, SP: prefusion S, SPG: prefusion S with VSV-G tail, r-SP: recombinant SP protein. Sera were collected 2 weeks after each injection. (a) Pooled sera from each group were analyzed for specific SARS-CoV-2 S (S1 + S2) total IgG; results are represented as EPT corresponding to the first dilution that gave an OD 3-fold above background. (b) Pooled sera from each group were analyzed in PRNT assay with a 90% threshold (PRNT90) as described in Material and Methods. A pool of human sera from COVID-19 convalescent patients with moderate disease (HCS) was used as reference. (c-d) Individual sera were analyzed in ELISA using recombinant SARS-CoV-2 S (S1 + S2) protein (c) or recombinant SARS-CoV-2 RBD protein (d). All 10 sera from Gag eVLP control group were negative in the same ELISA at PRNT at dilution of 1/500 and were not represented in fig. c and d. P values from Kruskall-Wallis test comparing groups are indicated in c and d. Serum from mice that received the negative control eVLP deprived of S were below the detection limit in both S1S2 and RBD ELISA and were not represented on graphs c and d.