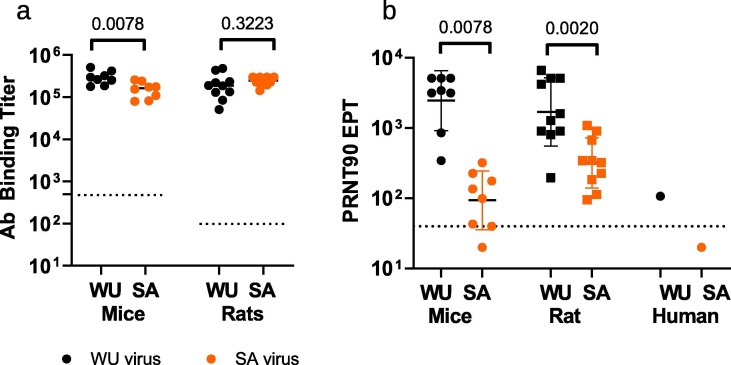
Fig. 5.
Crossreactivity induced by VBI-2902a on SARS-CoV-2 B.1.351 variant. Two groups of 8 mice received 2 injections of VBI-2902a or Gag eVLPs and two groups of 10 rats received 2 injections of VBI-2902a or Saline. Blood samples were collected 14 days after the second injection for monitoring of humoral responses. (a) Ab binding titers measured by ELISA using WU recombinant spike protein and SA variant recombinant spike protein as described in Material and Methods. All mouse sera from the Gag control group were negative at the first dilution tested of 1/500 and all rat sera from Saline control groups were negative above 1/100 dilution. These control values were represented by a doted line. (b) PRNT90 were performed as described in Material and Methods with either SARS-CoV-2 lineage B, hCOV-19/Canada/ON-VIDO-01/2020 (WU) or lineage B.1.351, hCOV-19/SouthAfrica/KRISP-EC-K005321/2020 (SA) viruses. GM with GM standard deviation are represented. A pool of COVID-19 convalescent human plasma samples (HCS) collected before emergence of the SA variant was also tested in PRNT90.The doted line represent the base line of the assay corresponding to the last dilution of 1/40. Values below this baseline indicated that no cytopathic effect was detected at the 1/40 dilution. Statistical analysis was performed with non parametric unpaired T test (Wilcoxon tests).