FIGURE 3.
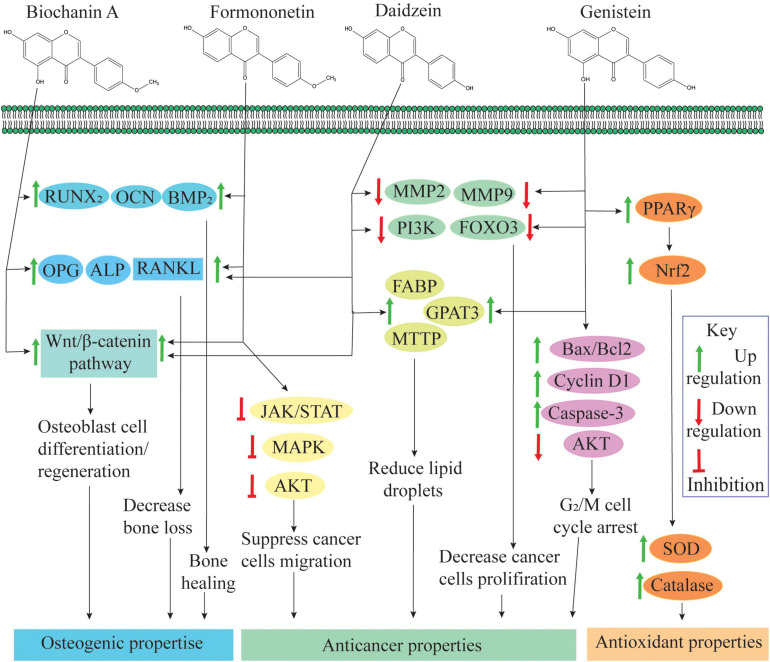
Role of isoflavonoids in human health: biochanin-A and formononetin are involved in bone healing and regeneration effects by upregulating runt-related transcription factor 2 (RUNX2), osteocalcin (OCN) and bone morphogenetic protein 2 (BMP2) expression at the injury site (Singh et al., 2017). Biochanin-A, formononetin, and daidzein also regulate osteoprotegerin (OPG), alkaline phosphatase (ALP), and receptor activator of nuclear factor κβ ligand (RANKL) expression; and these compounds are actively involved in osteogenic activities (Zakłos-Szyda et al., 2020). Additionally, an isoflavone mixture (biochanin-A, formononetin, and daidzein) promotes osteoblast cell differentiation and proliferation through the activation of the Wnt/β-catenin pathway (Chen et al., 2018). Additionally, formononetin inactivates signaling pathways, namely, Janus kinase/signal transducers, and activators of transcription (JAK/STAT) pathway, protein kinase B (PKB or AKT) pathway and mitogen-activated protein kinase (extracellular signal regulated kinase 1/2) [MAPK (ERK1/2)] pathways and suppresses cell migration, invasion and angiogenesis (Qi et al., 2016; Park et al., 2018). Daidzein and genistein can inhibit expression of matrix metalloproteinase-2/9 (MMP2/9), phosphatidylinositol-3-kinase (PI3K) and forkhead box O-3 (FOXO3) and reduce the cancer cell proliferation. Through activation of fatty acid-binding protein (FABP), glycerol-3-phosphate acyltransferase 3 (GPAT3) and microsomal triglyceride transfer protein (MTTP), genistein and daidzein reduce lipid droplet accumulation. Both of these situations induce apoptosis in cancer cells (as reviewed by Hsiao et al., 2020). Genistein activates the expression of Bcl-2-associated X-protein (Bax/Bcl-2), cyclin D1 and caspase-3 pathways and suppressed PI3K/AKT phosphorylation, which results in genistein-induced G2/M cell cycle arrest (Shafiee et al., 2016). Genistein activates peroxisome proliferator-activated receptor gamma (PPARγ) and enhances expression of superoxide dismutase (SOD) and catalase via Nrf2 activation (Chan et al., 2018).