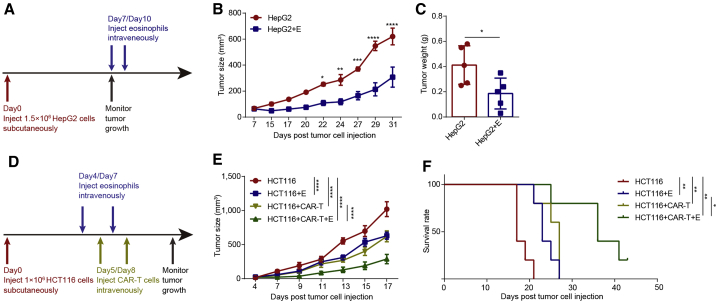
Figure 4.
Enhanced tumoricidal activity of CAR-T cells against solid tumors by combination with hESC-derived eosinophils
(A) Schematic showing the in vivo tumor assay.
(B) HepG2 tumor size of each group was determined on the indicated days after tumor cell injection (n = 5 for each group). Statistical significance was assessed using two-tailed ANOVA, where ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001; data are shown as the mean values ± SEM.
(C) Tumor weight of HepG2 on day 32 (n = 5 for each group). Statistical significance was assessed using unpaired t test, where ∗p < 0.05, data are shown as the mean values ± SD.
(D) Schematic showing the in vivo tumor assay of CAR-T cells and hPSC-derived eosinophils.
(E) HCT116 tumor size of each group was measured on the indicated days after tumor cell injection (n = 5 for control group, CAR-T cell group, and combination group; n = 4 for hESC-derived eosinophil group). Statistical significance was assessed using two-tailed ANOVA compared with the HCT116 group, where ∗∗∗∗p < 0.0001; data are shown as mean values ± SEM. These are representative data from two independent experiments.
(F) Kaplan-Meier curve representing the percentage survival of the experimental groups in the HCT116-xenograft mouse models (n = 5 for control group, CAR-T cell group, and combination group; n = 4 for hESC-derived eosinophil group). Statistical analyses were calculated using log-rank (Mantel-Cox) test, where∗p < 0.05 and ∗∗p < 0.01. These are representative data from two independent experiments.
See also Figures S3 and S4.