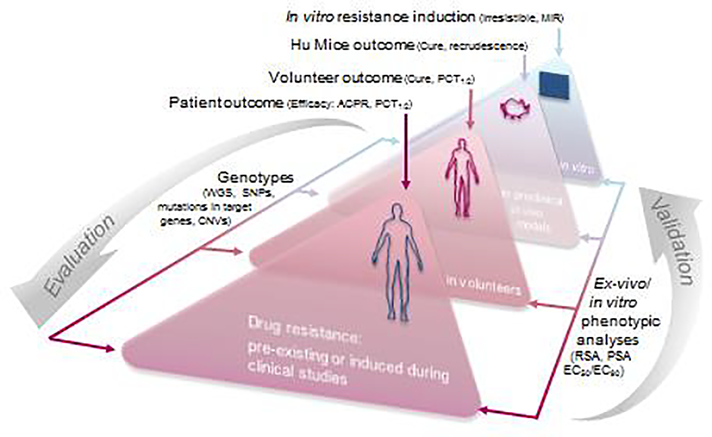
Figure 2. Resistance risk validation and evaluation, an iterative process between the bedside and the bench.
Learning from the clinical observations collected over the past two decades, our proposed new strategy for the evaluation of resistance risks relies on the “resistance triangle”. First, clinical outcomes, phenotypic studies and genotypic analyses are compiled from P. falciparum-infected patients and volunteers, through in vivo models, down to in vitro experiments. Resistance is declared when parasites from clinical recrudescences harbor validated resistant genetic markers and exhibit decreased in vitro sensitivity to the drug/compound. ACPR: adequate clinical and parasitological response; PCT: parasite clearance time; MIR: minimum inoculum for resistance; WGS: whole-genome sequencing; SNP: single nucleotide polymorphism; CNV: copy number variation; RSA: ring-survival assay, PSA: piperaquine survival assay.