Fig. 4. Synthetic applications of pyrrolidine derivative 2s.
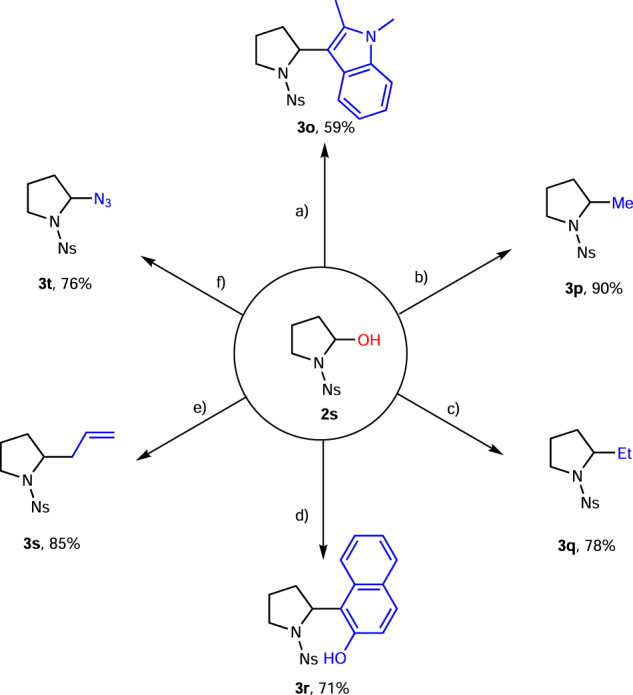
Reaction conditions: 2s (0.1 mmol), CH2Cl2 (1.0 mL), BF3·OEt2 (0.2 mmol), and nucleophile (0.3 mmol) added at −78 °C for 1 h; rt for 3 h. (a) 1,2-Dimethylindole as a nucleophile and the reaction was performed at −78 °C for 3 h. (b) AlMe3 as a nucleophile. (c) Et2Zn as a nucleophile. (d) 2-Naphthol as a nucleophile. (e) Allyltributylstannane as a nucleophile. (f) TMSN3 as a nucleophile.
