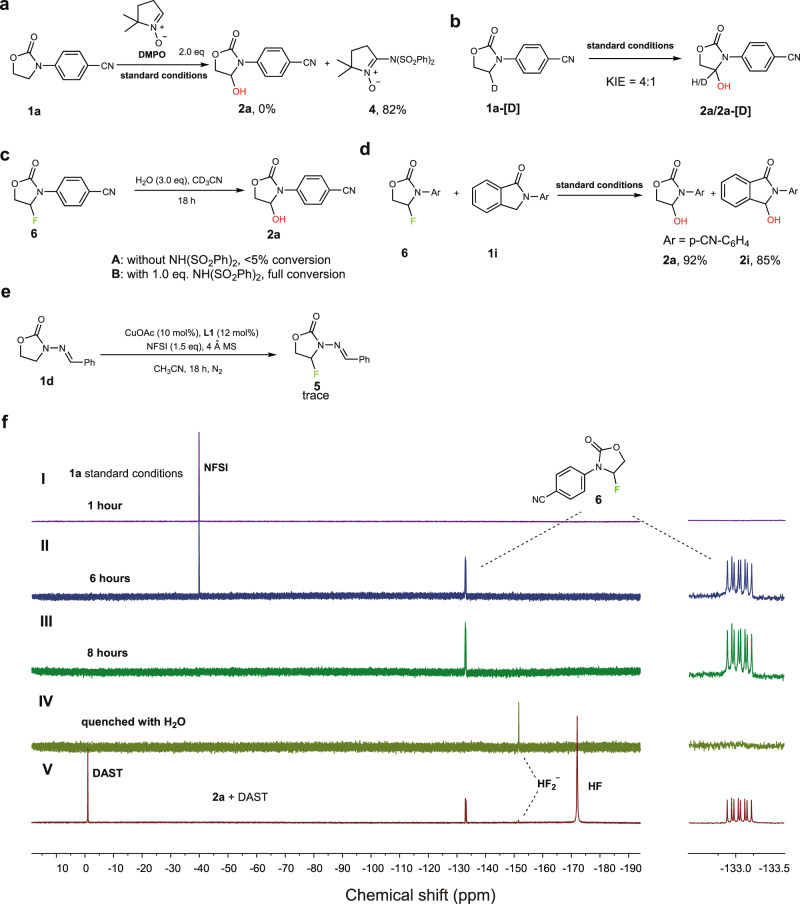
Fig. 6. Mechanistic experiments.
a Free-radical scavenging experiment. b KIE experiment. c Conditions: CD3CN (0.5 mL), 6 (0.1 mmol), and H2O (0.3 mmol). d Cross-over experiment of the intermediate 6 and 1i under the standard conditions. e Conditions: in glovebox, 4 Å molecular sieve (0.5 g), CH3CN (1.0 mL), 1d (0.1 mmol), CuOAc (0.01 mmol), ligand 1 (0.012 mmol), and NFSI (0.15 mmol). f 19F (376 MHz) NMR spectra of the N-heterocyclic C–H functionalization reaction. Conditions: CD3CN (0.5 mL), 1a (0.1 mmol), CuOAc (0.01 mmol), ligand 1 (0.012 mmol), and NFSI (0.15 mmol). (I) 19F (376 MHz) NMR spectrum of the N-heterocyclic C–H functionalization reaction after 1 h. (II) 19F (376 MHz) NMR spectrum of the reaction after 6 h. (III) 19F (376 MHz) NMR spectrum of the reaction after 8 h. (IV) 19F (376 MHz) NMR spectrum of the reaction after adding 0.01 mL H2O. (V) Fluorinated intermediate 6 prepared from 2a and DAST: CD3CN (0.5 mL), 2a (0.1 mmol), and DAST (0.1 mmol) .19F (376 MHz) NMR spectrum of the reaction after 1 h.