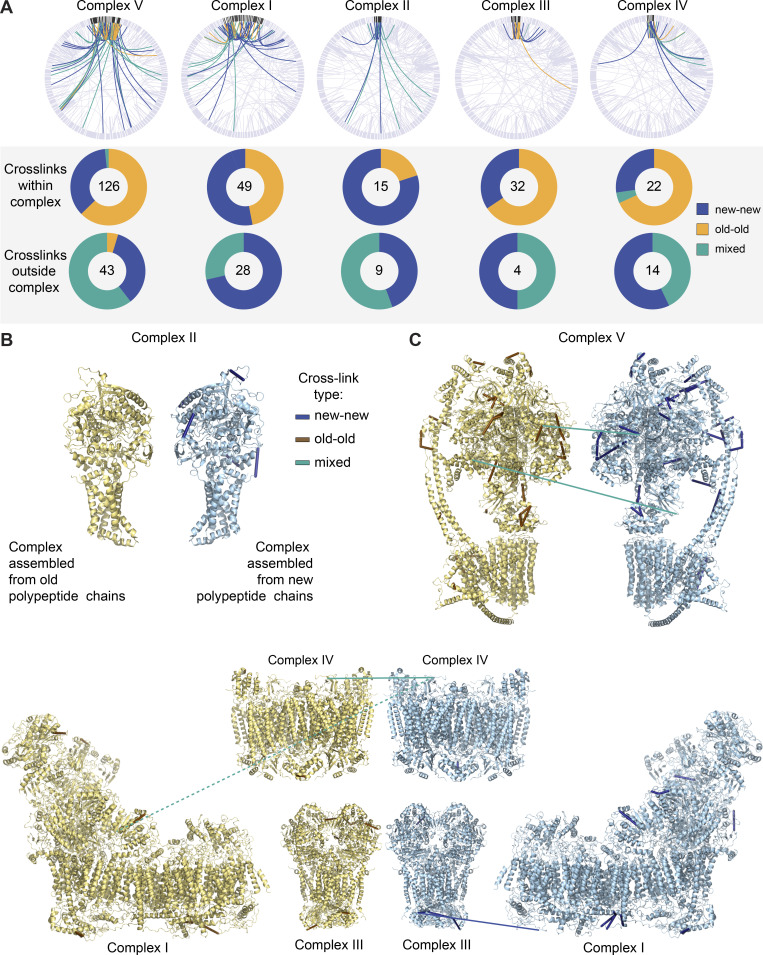
Figure 5.
OXPHOS complexes are copreserved with limited subunit exchange for months. (A) Total cross-links within individual OXPHOS complexes are predominantly between two peptides of the same isotope build (new-new or old-old). Cross-links between OXPHOS complexes and other mitochondrial proteins are mostly between new-new or new-old peptides but are rarely formed between two old peptides. (B) Inter- and self- (nonoverlapping peptides) cross-links identified in new and old complex II mapped to high-resolution protein structures (Protein Data Bank [PDB] accession no. 1ZOY). No cross-links were identified between old-old or old-new complex II proteins. Yellow structure illustrates complex build from old, 14N, peptides; blue structure illustrates complex build from new, 15N peptides. (C) Inter- and self- (nonoverlapping peptides) cross-links identified in new and old complex V mapped to high-resolution protein structures (PDB accession no. 6J5I). Only two cross-links of mixed isotypes were identified (teal). (D) PDB structure of complexes I (accession no. 6G2J), III (accession no. 1BGY), and IV (accession no. 3X2Q), which can assemble into respirasome supercomplexes. Inter- and self- (nonoverlapping peptides) cross-links between new or old proteins, as well as intercomplex cross-links, are shown. Blue indicates cross-links between two new peptides, and teal represents cross-links between new and old peptides. Dotted line represents a cross-link between complex I and IV, which is outside of cross-linker constraints. Maps were generated in XIView. n = 2 mice. See also Fig. S6.