Fig 2.
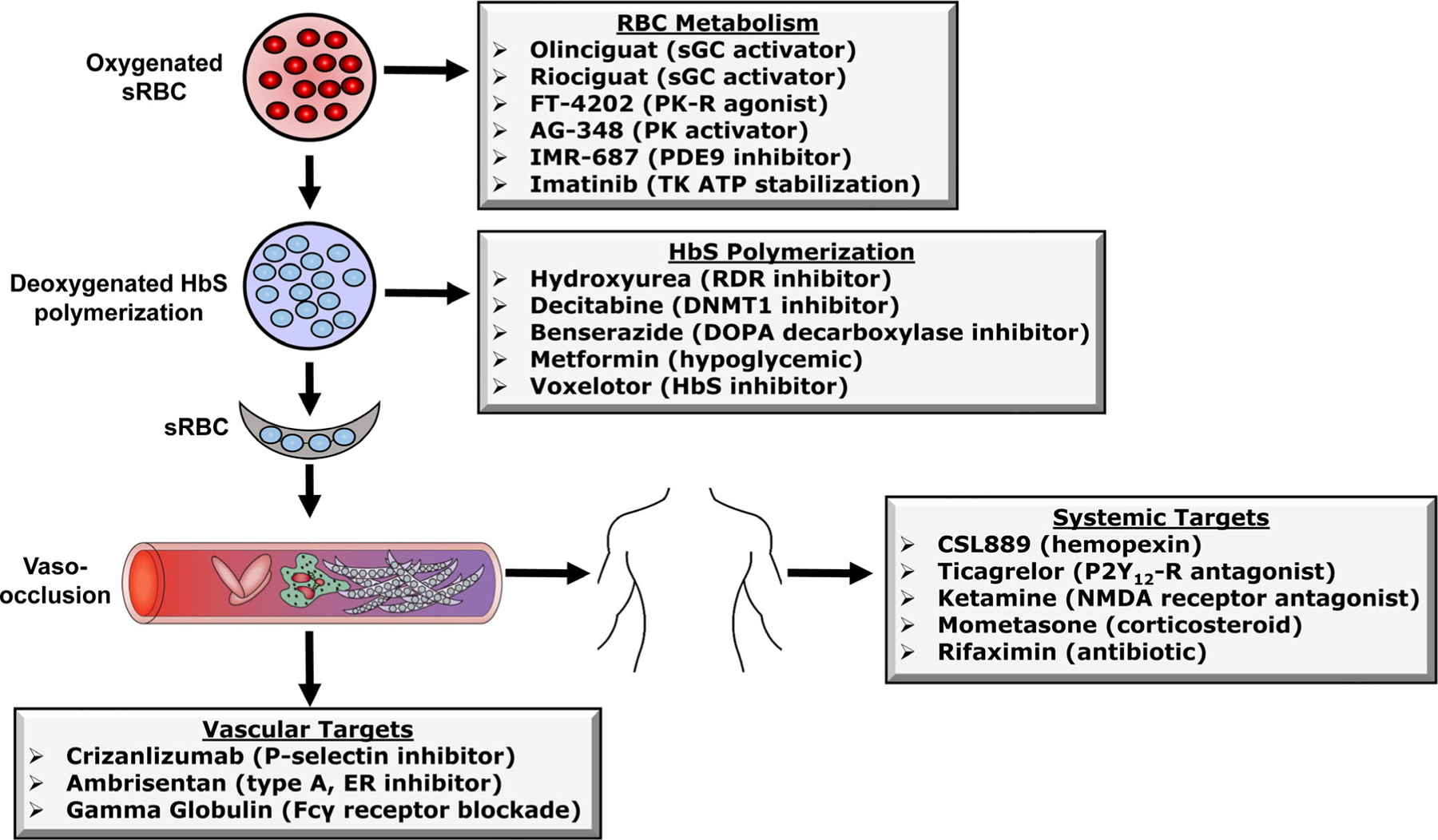
Mechanisms of action of drugs under evaluation in human clinical trials. Shown is a summary of the major cellular, vascular and system mechanisms by which FDA-approved and other drugs improve the clinical severity of sickle cell disease. The effects have been divided into four areas/targets as shown for the sake of discussion in the text. Abbreviations: sRBC, sickle red blood cells; HbS, haemoglobin S; sGC, soluble guanylyl cyclase; PK-R, pyruvate kinase-R; PK; pyruvate kinase; TK, BCR/ABL tyrosine kinase ATP tyrosine kinase, RDR, ribonucleoside diphosphate reductase; DNMT1, DNA methyltransferase 1; DOPA, dihydroxyphenylalanine; ER, endothelial receptor; NMDA, N-Methyl-d-aspartic acid or N-Methyl-d-aspartate; P2YR, purinergic.
