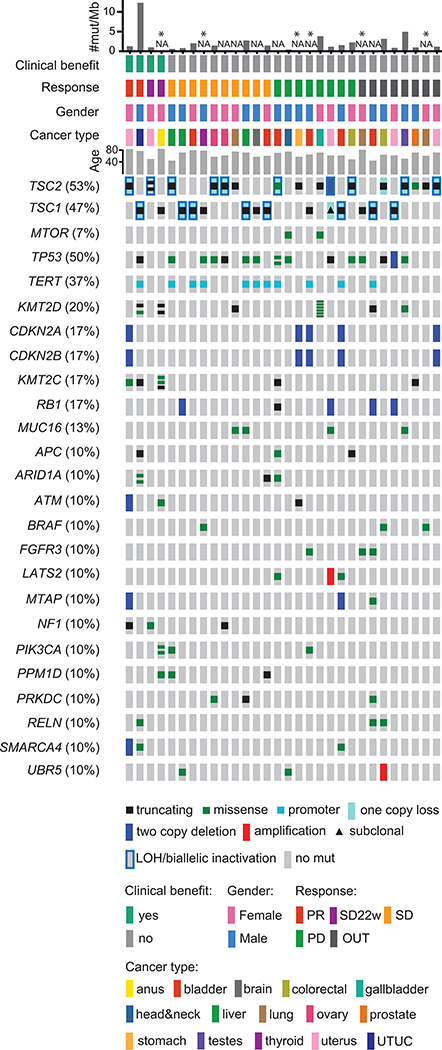
Fig 3. Co-mutation plot of all variants.
SNVs/indels, LOH/biallelic inactivation events for TSC1 and TSC2, two-copy deletions, and amplification events are shown for cancer genes in the OncoKB and/or CGC databases, for which at least 10% of subjects had an event. For TSC1 and TSC2, only inactivating mutations are shown with indication of subclonal mutation. Clinical benefit was defined as PR: partial response, or stable disease at 22w with minimal reduction (SD22w); SD - stable disease, PD - progressive disease, and OUT - subject drop-out. Tumor mutation burden (TMB) for each patient is shown at top as nonsynonymous SNVs/indels per megabase of targeted capture genomic region. TMB is shown only for patients with Tumor-Normal paired WES analysis; patients with no WES available (results of MPS targeted assay only) are indicated with an asterisk. Abbreviations: UTUC – upper tract urothelial carcinoma.