Figure 5.
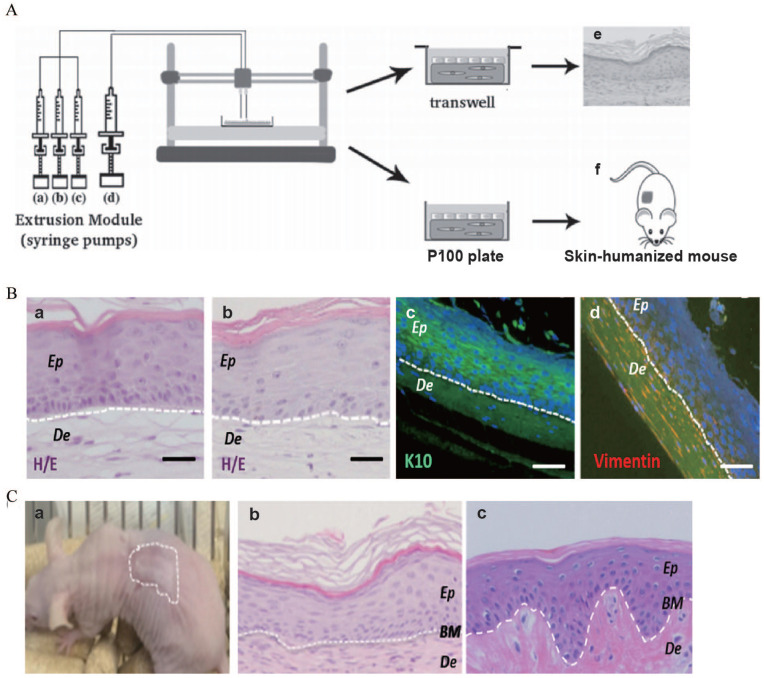
(A) Scheme of the bioprinting process: The extrusion module contained four syringes, loaded with (a) hFbs, (b) plasma, (c) CaCl2, (d) hKCs, respectively, (e) equivalents printed on transwell inserts could differentiate at the air–liquid surface for 17 days, and (f) equivalents printed on P100 plates were grafted on to the backs of immunodeficient mice for 8 weeks. (B) In vitro 3D human skin equivalents obtained after 17 days of differentiation at the air–liquid interface: (a) “handmade” skin equivalent following our previous protocol, (b–d) printed skin equivalents, (a and b) H/E staining, (c and d) immunostaining using an anti-K10 antibody (c) and an anti-human vimentin antibody (d). Ep and De in (a–d) denote the epidermal and the dermal compartments, respectively. (C) Histological analysis (8 weeks postgrafting) of bioprinted human skin grafted to immunodeficient mice: (a) visual appearance of the grafted human skin, (b) H/E staining of the regenerated human skin, (c) H/E staining of normal human skin. The white dotted line in (b) and (c) indicates the dermo-epidermal junction (basal membrane, BM). Scale bar: 100 μm.
Source: Adapted with permission from Cubo et al. 163