Summary
Mutations in microglia may cause brain disorders. Replacement of dysfunctional microglia by allogeneic wild-type microglia from bone marrow transplantation (Mr BMT) or peripheral blood can correct the gene deficiency at the brain-wide scale but cannot achieve precise replacement at specific brain regions. Here, we introduce a strategy with potential clinical relevance—microglia replacement by microglia transplantation (Mr MT), combining tamoxifen-induced ablation of Mr BMT cells and intracranial injection of microglia to mouse brain, to achieve region-sepcific microglia replacement. The original abbreviation of this microglia replacement strategy is mrMT. We hereby change the name to Mr MT.
For complete details on the use and execution of this protocol, please refer to Xu et al. (2020).
Subject areas: Cell Biology, Immunology, Microscopy, Model Organisms, Neuroscience, Stem Cells
Graphical abstract

Highlights
-
•
Protocol describes tamoxifen-induced ablation of the Mr BMT cells
-
•
Procedures for intracranial injection of microglia to mouse hippocampus
-
•
Mr MT achieves microglia replacement/transplantation at the brain region of interest
-
•
Mr MT cells exhibit the microglia-like characteristics
Mutations in microglia may cause brain disorders. Replacement of dysfunctional microglia by allogeneic wild-type microglia from bone marrow transplantation (Mr BMT) or peripheral blood can correct the gene deficiency at the brain-wide scale but cannot achieve precise replacement at specific brain regions. Here, we introduce a strategy with potential clinical relevance—microglia replacement by microglia transplantation (Mr MT), combining tamoxifen-induced ablation of Mr BMT cells and intracranial injection of microglia to mouse brain, to achieve region-sepcific microglia replacement. The original abbreviation of this microglia replacement strategy is mr MT. We hereby change the name to Mr MT.
Before you begin
Prepare the PLX5622-formulated AIN-76A chow diet
Prepare following the below recipe: 1.2 g PLX5622 per kg AIN-76A diet. The PLX5622 chow was formulated by SYSY Bio. One adult mouse (25 g) consumes about 3.5 g chow diet per day.
Store at −20°C before use. The PLX5622-formulated diet is valid for at least one year.
Prepare the tamoxifen-formulated chow diet
Prepare following the below recipe: 400 mg tamoxifen citrate and 49.6 g sucrose per kg of Teklad Global 16% Protein Rodent Diet (2016) (Envigo, TD.130860).
Store at −20°C before use. The tamoxifen-formulated diet is valid for at least one year.
Prepare CX3CR1+/GFP mice
Cross CX3CR1GFP/GFP with C57BL/6J to obtain the CX3CR1+/GFP mice.
Prepare CX3CR1-CreER::DTA mice
Cross CX3CR1CreER/CreER with Rosa26-DTA to obtain the CX3CR1+/CreER;Rosa26-DTAwt/mut (CX3CR1-CreER::DTA) mice.
Prepare acid water
Prepare 2 L drinking water and adjust pH to 2 to 3 by 0.1 M HCl. Autoclave the acidified water. After the water is cooled to room temperature, add 2.2 g neomycin and thoroughly mix the solution. Store the acid water at 4°C before use. The acid water is valid for at most half a year.
Key resources table
| REAGENT or RESOURCES | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| PLX5622 | MedChemExpress (MCE) | HY-114153 |
| PLX5622-formulated AIN-76A diet | SYSE Bio | N/A |
| AIN-76A control diet (CD) | SYSE Bio | N/A |
| Tamoxifen-formulated AIN-76A diet | Envigo | TD.130860 |
| Tamoxifen citrate | Aladdin | T101373 |
| L15 medium | Thermo Fisher | 11415064 |
| DPBS | Thermo Fisher | 14190144 |
| Collagenase, Type II | Thermo Fisher | 17101015 |
| 10× PBS | Thermo Fisher | 70011069 |
| ACK lysis buffer | Thermo Fisher | A1049201 |
| Papain | Sigma-Aldrich | 76220 |
| Cysteine | Sigma-Aldrich | 168149 |
| Low endotoxin bovine serum albumin, fraction V | Sangon | A602442 |
| Ovomucoid | Sangon | A003085 |
| DNase I | Sangon | A610099 |
| Heparin sodium | Sangon | A603251 |
| CD11b MicroBeads, human and mouse | Miltenyi | 130-049-601 |
| Percoll | Solarbio | P8370 |
| DMEM/F12 | Thermo Fisher | 21041-02 |
| Glutamine | Gibco | 25030-081 |
| N-acetyl cysteine | Sigma | A9165 |
| Insulin | Sigma | 16634 |
| Apo-transferrin | Sigma | T1147 |
| Sodium selenite | Sigma | S-526 |
| Heparan sulfate | Galen Laboratory Supplies | GAG-HS0 |
| TGF-β2 | PeproTech | 100-35B |
| Murine IL-34 | R&D Systems | 5195-ML/CF |
| Ovine wool cholesterol | Sigma | C8667 |
| Oleic acid | Sigma | O1383 |
| Gondoic acid | Sigma | E3635 |
| DMSO | Sigma | D2650 |
| Neomycin | Sigma | N6386-100G |
| Meloxicam | Sigma | M3935 |
| Ketamine | Guyao | N.A. |
| Xylazine | Sigma | X1251 |
| Experimental models: organisms/strains | ||
| C57BL/6J | Charles River (Beijing Vital River Laboratory Animal Technology) | C57BL/6Jnifdc, Stock No.: 219 |
| Rosa26-DTA | The Jackson Laboratory | B6.129P2-Gt(ROSA)26Sor tm1(DTA)Lky /J, Stock No.: 009669 |
| CX3CR1CreER/CreER | The Jackson Laboratory | B6.129P2(C)-CX3CR1tm2.1(cre/ERT2)Jung/J, Stock No.: 020940 |
| CX3CR1GFP/GFP | The Jackson Laboratory | B6.129P-CX3CR1tm1Litt/J, Stock No: 005582 |
| Other | ||
| MACS Separation Columns (LS) | Miltenyi | 130-042-401 |
| QuadroMACS Separator 76 | Miltenyi | 130-090-976 |
| MACS MultiStand | Miltenyi | 130-042-303 |
| Refrigerated centrifuge | Eppendorf | 5804R |
| Rotor with buckets | Eppendorf | S-4-72 |
| Vacuum pump | Kylin-Bell | GL-802B |
| Incubated shaker | Shanghai Yiheng | THZ-100 |
| Mouse injection cone with restrainer and LED | GLOBALEBIO | GEGD-Q9G |
| Stereotactic apparatus | RWD | 68045 |
| Micropump | LONGER | TJ-1A |
| Cell strainer | Falcon | 352340 (40 μm pores) 352360 (100 μm pores) |
| Scalpel blades | Jinhuan | 11 |
| Scalpel holder #3 | FST | 10003-12 |
| Hot bead sterilizer | FST | 18000-45 |
| Iodophor | LIRCON | DFXDY |
Materials and equipment
Perfusion solution (4°C, for at most half a year)
| Reagent | Amount |
|---|---|
| 1× PBS (0.01 M) | 500 mL |
| Heparin sodium | 1,000 U |
| Total | 500 mL |
Enzyme Digestion Mix (prepare right before use)
| Reagent | Amount |
|---|---|
| 1× L15 medium | 10 mL |
| Papain | 165 U |
| Cysteine | 2 mg |
| Collagenase II | 1,000 U |
| DNase I | 80 k unit |
| NaOH | Adjust to pH 7.2 |
| Total | 10 mL |
Trituration buffer (4°C, for at most half a year)
| Reagent | Amount |
|---|---|
| L15 medium | 500 mL |
| BSA | 0.5% (w/v) |
| Total | 500 mL |
90% percoll solution (4°C, for at most half a year)
| Reagent | Amount |
|---|---|
| 1× PBS (0.01 M) | 5 mL |
| Percoll | 45 mL |
| Total | 50 mL |
Percoll working buffer (prepare right before use)
| Reagent | Amount |
|---|---|
| Trituration buffer | 30 mL |
| 90% percoll | 15 mL |
| Total | 45 mL |
MACS Buffer (4°C, for at most half a year)
| Reagent | Amount |
|---|---|
| 1× PBS (0.01 M) | 500 mL |
| BSA | 0.5% (w/v) |
| Total | 500 mL |
10× Papain neutralization solution (−20°C, for at most half a year)
| Reagent | Amount |
|---|---|
| Ovomucoid | 500 mg |
| 1× PBS (0.01 M) | 25 mL |
| NaOH | Adjust to pH 7.2 |
| Total | 25 mL |
Microglia re-suspension solution
Make the following stock:
| Stock reagents | Stock concentration | Working dilution | Storage |
|---|---|---|---|
| Apo-transferrin | 10 mg/mL in PBS | 1:100 | −20°C, for at most half a year |
| N-acetyl cysteine | 5 mg/mL in H2O | 1:1,000 | −20°C, for at most half a year |
| Sodium selenite | 2.5 mg/mL in H2O | 1:25,000 | −20°C, for at most half a year |
| TGF-b2 | 2 mg/mL in PBS | 1:1,000 | −20°C, for at most half a year |
| IL-34 | 200 μg/mL in PBS | 1:1,000 | −80°C, for at most half a year |
| Insulin | 500 μg/mL in PBS | 1:100 | −20°C, for at most half a year |
| Ovine wool cholesterol | 1.5 mg/mL in 100% ethanol | 1:1,000 | −20°C, for at most half a year |
| Heparan sulfate | 1 mg/mL in H2O | 1:1,000 | −20°C, for at most half a year |
| Oleic acid | 1 mg/mL in DMSO | 1:10,000 | −20°C, for at most half a year |
| Gondoic acid | 0.01 mg/mL in DMSO | 1:10,000 | −20°C, for at most half a year |
Microglia re-suspension solution recipe (−20°C, for at most half a year):
| Reagent | Amount |
|---|---|
| DMEM/F12 | 10 mL |
| Glutamine (200 mM) | 100 μL |
| N-acetyl cysteine stock | 10 μL |
| Insulin stock | 100 μL |
| apo-transferrin stock | 100 μL |
| sodium selenite stock | 0.4 μL |
| TGF-b2 stock | 10 μL |
| IL-34 stock | 10 μL |
| heparan sulfate stock | 10 μL |
| oleic acid stock | 1 μL |
| gondoic acid stock | 1 μL |
| cholesterol stock | 10 μL |
| Total | 10 mL |
CRITICAL: Warm the medium to 37°C before adding the oleic acid stock, gondoic acid stock, and cholesterol stock. Do not filter the cholesterol-containing medium.
Step-by-step method details
The overall procedure is outlined in Figure 1.
Figure 1.
Scheme of Mr MT
Mr MT procedure consists of two steps: the first step is replacing the recipient’s endogenous microglia by tamoxifen-sensitive CX3CR1-CreER::DTA cells by microglia replacement by bone marrow transplantation (Mr BMT) (refer to STAR Protocols STAR-PROTOCOLS-D-21-00166R2). After that, transplant the donor microglia intracranially at day 44. Tamoxifen is continuously applied to kill the endogenous Mr BMT cells without affecting the transplanted microglia.
WBI: whole-body irradiation; OG: oral gavage; BMT: bone marrow transplantation; MT: microglia transplantation; PLX5622: PLX5622-formulated AIN-76A diet; CD: control diet (AIN-76A diet).
Replace endogenous microglia of the C57BL/6J mouse with gender-matched CX3CR1-CreER::DTA microglia-like cells through mr BMT
Timing: 44 days
The first step of Mr MT is replacing the endogenous microglia with tamoxifen-sensitive CX3CR1-CreER::DTA microglia-like cells through Mr BMT (Xu et al., 2020). For the detailed protocol of Mr BMT, please kindly refer to Xu et al. (2021).
Note: The only difference is the donor cells used. For this protocol, utilize the CX3CR1-CreER::DTA mouse as the donor. In step 8 of the Mr BMT STAR protocols paper, we use the gender-matched CX3CR1+/GFP mouse. But for Mr MT, we use the gender-matched CX3CR1-CreER::DTA mouse here.
Tamoxifen-induced ablation of the CX3CR1-CreER::DTA mr BMT cells
Timing: 4 days
-
1.
From D41 to D44, three days before the intracerebral injection (Figure 1), treat the recipient mouse with tamoxifen (150 mg/kg body weight, dissolved in olive oil) through an oral gavage for 4 consecutive days to initiate the ablation of CX3CR1-CreER::DTA Mr BMT cells (Figure 2).
Figure 2.
Microglia depletion in CX3CR1-CreER::DTA mice via tamoxifen administration
Microglia are revealed by IBA1 immunostaining
(A) IBA1 positive cells before tamoxifen treatment.
(B) IBA1 positive cells after the tamoxifen treatment for 10 days (without transplanation).
Microglia isolation from the CX3CR1+/GFP mouse
Brain isolation
Timing: 30 min
-
2.
Turn on the orbital shaker incubator, set the temperature at 35°C and speed at 100 rpm. Preheat the incubator for at least 20 min to 35°C. This temperature helps reduce cell death during digestion.
-
3.
Aliquot 4 mL of Enzyme Digestion Mix per brain into a 15 mL centrifuge tube.
-
4.
Deeply anesthetize the adult CX3CR1+/GFP mouse (>2 months old, gender-matched to the recipient mouse) with ketamine (100 mg/kg, dissolved in saline) and xylazine (10 mg/kg, dissolved in saline). Pinch the tail to ensure the mouse is in a deep anesthetized state before the transcardial perfusion. Otherwise, it will cause tremendous suffering to the mouse.
-
5.
Transcardially perfuse the donor mouse with ice-cold perfusion solution (L15 medium + 3 U/L heparin) until the skull turns white.
-
6.
Decapitate the mouse and put the head in the ice-old PBS for 1 min. Make small incisions laterally on either side at the caudal/ventral base of the skull. Make additional shallow cuts starting at the caudal/dorsal aspect of the skull moving in the rostral direction up the dorsal midline. Make a final “T” cut perpendicular to the midline at the level of the olfactory bulbs. Use the round-tip forceps to grasp the skull starting at the rostral-medial aspect and peel back towards the caudal-lateral direction. Repeat for both sides to crack open and remove the dorsal halves of the skull cap to expose the brain. Harvest the brain and wash it with fresh ice-cold PBS. The brain should be white without the presence of blood (Figure 3).
CRITICAL: All reagents and surgical tools should be ice-cold. The operation should be quick to minimize the damage to the cells.
Figure 3.
Harvest the brain after perfusion
(A1–A5) The procedure of the skull removal for brain harvest.
(B) The harvested brain. Note that no blood vessels can be seen.
Brain digestion
Timing: 22 min
-
7.
Chop the brain using a razor blade on ice with 100 μL ice-cold PBS into ∼1 mm pieces. Then place the minced brain tissue into 3 mL enzyme digestion mix in a 15 mL conical centrifuge tube.
-
8.
Shake the minced brain in the orbital shaker incubator at 35°C, 100 rpm for 20 min (Methods video S1).
-
9.
Terminate the digestion by adding 0.5 mL 10× papain neutralization solution. Gently mix and sit on ice for about 1 min for tissue to settle (Figure 4A).
Figure 4.
Trituration of the brain tissue
(A) Terminate the digestion by adding 0.5 mL 10× papain neutralization solution; gently mix and sit still for about 1 min for tissue to settle.
(B) Rinse the 100 μm cell strainer with DPBS and insert it into a 50 mL centrifuge tube.
(C) Transfer the supernatant above the brain chops in (A) to the 50 mL tube containing the strainer in (B), and leave the brain chops in the original tube.
(D) Add 2 mL trituration solution to the 15 mL tube containing the brain chops; gently triturate the brain chops 10 times with a 1 mL tip pipette, and put on the ice for about 1 min.
(E) Repeat B to D until the minced brain is diminished to about 3 mm in height.
Trituration
Timing: 10 min
-
10.
Rinse the 100 μm cell strainer with DPBS and insert it into a 50 mL centrifuge tube (Figure 4B). Cool it in ice.
-
11.
Transfer the supernatant above the brain chops (Figure 4A) to the 50 mL tube containing the strainer, and leave the brain chops in the original tube (Figure 4C).
-
12.
Add 2 mL trituration solution to the 15 mL tube containing the brain chops (Figure 4D).
-
13.
Gently triturate the brain chops 10 times with a 1 mL tip pipette, and put on the ice for about 1 min (Figure 4A).
-
14.
Repeat steps 11 to 13 until the minced brain is diminished to about 3 mm in height. Discard the remanence (Figure 4E).
Cell debris removal by percoll gradient centrifuge
Timing: 30 min
-
15.
Precool the centrifuge to 18°C.
-
16.
Centrifuge the brain at 400 g, 18°C for 5 min.
-
17.
Insert a 10 μL white tip on the metal pipe of the vacuum pump to aspirate the supernatant with vacuum pump suction (Figure 5).
-
18.
Add Percoll working solution to the pellet and mix gently until the pellet disappears. Then transfer the cell suspension to a new 15 mL tube.
-
19.
Centrifuge the tissue at 400 g, 18°C for 20 min (set the acceleration and deceleration speed to zero). Set the centrifuge temperature to 4°C after the centrifugation finished
CRITICAL: The temperature must be set to 18°C.
-
20.
The myelin debris is separated from living cell pellets (Figure 6). Carefully take out the tube containing the cell pellets from the centrifuge.
-
21.
Insert a 200 μL yellow tip on the metal pipe of the vacuum pump. Carefully aspirate the myelin debris and supernatant by the vacuum pump suction (Methods video S2). Add 5 mL trituration solution and wash by centrifugation at 4°C 400 g for 5 min.
Figure 5.
Aspirate the supernatant from the brain pellet through a vacuum pump
Figure 6.
Separation of myelin debris from cell pellets in 30% Percoll
Microglia purification by magnetic-activated cell sorting (MACS)
Timing: 1 h
-
22.
Add 10 μL anti-CD11b beads into 90 μL trituration solution. Mix thoroughly.
-
23.
Re-suspend the cell pellet with the above mixture.
-
24.
Incubate the cell suspension at 4°C for 15 min. Shake the cell gently every 5 min.
-
25.
Wash the cell suspension with 5 mL MACS buffer through 400 g centrifugation at 4°C for 5 min.
-
26.
Re-suspend the cell pellet with 3 mL MACS buffer, filter the cell suspension through a 40 μm pore cell strainer.
-
27.
Setup the LS column on a MACS multistand.
-
28.
Wash the LS column by 3 mL MACS buffer twice.
-
29.
Immediately pour all the cell suspension into the column
-
30.
Once liquid has finished flowing through the columns, wash the column twice with 3 mL MACS buffer.
CRITICAL: Do not let the column dry.
-
31.
Remove the column from the stand and insert it into a new 15 mL centrifuge tube. Add 5 mL MACS buffer in the column and flush the column as quick as possible using the supplied plunge.
-
32.
Centrifuge at 400 g, 4°C for 10 min. The cell pellet will appear at the tube bottom (Figure 7).
Optional: Repeat steps 26 to 32 if additional microglial purification is required.
-
33.
Re-suspend the cell with 100 μL microglia resuspension solution; transfer the cell suspension to a 200 μL tube. Keep the cell suspension on ice until use.
-
34.
Take 1 μL out of the suspension and dilute it to 100 μL. Count the cell number with a cell cytometry. The common output is about 2 × 105. The purity of GFP-positive cell is typically 80%.
CRITICAL: The cell suspension must be transferred to the 200 μL tube.
Note: To get a higher cell purification, we usually perform the fluorescence activated cell sorting (FACS) by gating the GFP-positive cells.
Figure 7.
Cell pellet (in red circle) after MACS
Stereotactic injection at the unilateral hippocampus
Timing: 40 min
-
35.
Clean the operation bench with 75% ethanol.
-
36.
Turn on the FST hot bead sterilizer and heat the beads to about 250°C. Insert surgical tools (fine scissors, dressing forceps, needle holders) deep into the beads for at least 20 s. Put the sterilized surgical tools on the sterilized towel paper.
-
37.
Intraperitoneally inject the recipient mouse (C57BL/6J that received bone marrow transplantation from Cx3cr1-CreER:DTA mice, age and gender-matched to the donor mouse) with the mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). After the mouse loses refectory response to toe pinching, shave the scalp hair with scissors and apply depilatory cream on the scalp. Two minutes later, clean the scalp with water.
-
38.
Fix the mouse head on a stereotactic apparatus. Apply iodophor on the scalp with a cotton swab and cut the scalp by scissors to exposure the skull. Use cotton swab to remove the mucosa over the skull till the skull gets dry.
-
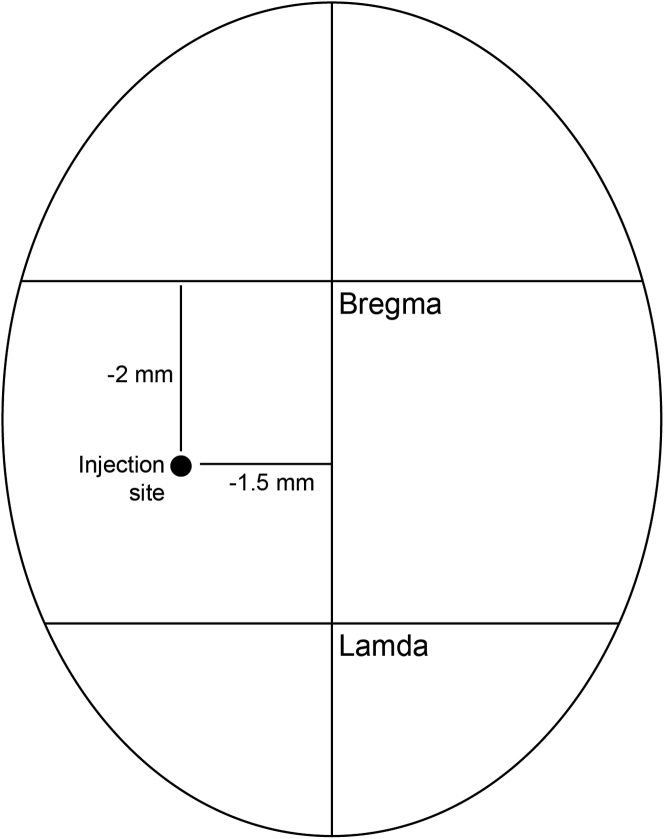
39.
Mark the following location on the skull: −2.00 mm at AP and −1.5 mm at ML with an 18 G needle (Figure 8).
-
40.
Moisten the skull surface by DPBS. Use a microdrill with a 0.6 mm bore bit to open a dimple at the marked location, using the drill speed at 200 rpm. Carefully remove the thinned skull piece and the underneath dura in the dimple with a 27 G needle.
CRITICAL: Moistening is key to visualizing major brain surface vasculature. Avoid bleeding and damaging the brain tissue by avoiding stabbing the major vasculature.
-
41.
Concentrate the microglia by centrifugation at 100 g for 1 min. Discard the supernatant. Use a cell counter to calculate the amount of microglia resuspension solution (usually with remaining volume less than 5 μL). Control the cell concentration to 1 × 105 cells per μL. Aspirate 0.8 μL concentrated cell suspension into a pre-cooled 5 μL Hamilton syringe equipped with a 30 G needle. Attach the syringe on a micro-pump and set the following injection parameter: injection amount, 0.3 μL; injection speed, 3 min. Connect the syringe with the pump onto the arm of the stereotactic apparatus.
-
42.
Move the syringe needle close to the small window on the skull. Insert the needle to the following depths: −1.5 mm and −0.7 mm of the unilateral brain. Start the injection process and infuse 0.3 μL microglia cell suspension at each depth. At the end of the second infusion, hold the needle in place for 1 min, and then slowly withdraw the needle at about 0.02 mm/s.
-
43.
Moisten the skull and the scalp with DPBS. Close the scalp together with 4 to 5 sutures, 3 mm apart. Sterilize the suture with iodophor and apply erythromycin ointment on the wound.
-
44.
Intraperitoneally inject 0.5 mL sterilized saline to the mouse. Put the mouse into a cage laid with sterilized towel paper. Put the cage on a heating pad set at 45°C. Put a few moistened chows near the mouse. Add neomycin into the drinking water (1.1 g/L). Wait till until the mice recovered from anesthesia.
-
45.
Inject meloxicam (1 mg/kg, disolved in saline) intraperitoneally. Put the cage back to the animal facility.
Figure 8.
Illustratration of injection site on the mouse skull
Post-operational care
-
46.
Inject meloxicam (1 mg/kg) intraperitoneally every 12 h for the first 2 days after the surgery.
-
47.
Change the bedding materials every day and keep the cage clean.
Note: We kept these operated animals outside the SPF environment. However, it is preferable to maintain the moue in SPF environment.
-
48.
Check for any distressing behavior of the mice. If the mice lose 20% of body weight abruptly in a week, ask help from the veterinarian and considering euthanasia.
-
49.
Feed the mouse with tamoxifen-formulated chow diet ad libitum for 30 days. Upon tamoxifen treatment, the CX3CR1-CreER::DTA cells are continuously killed and suppressed while the injected CX3CR1+/GFP microglia are not affected.
Expected outcomes
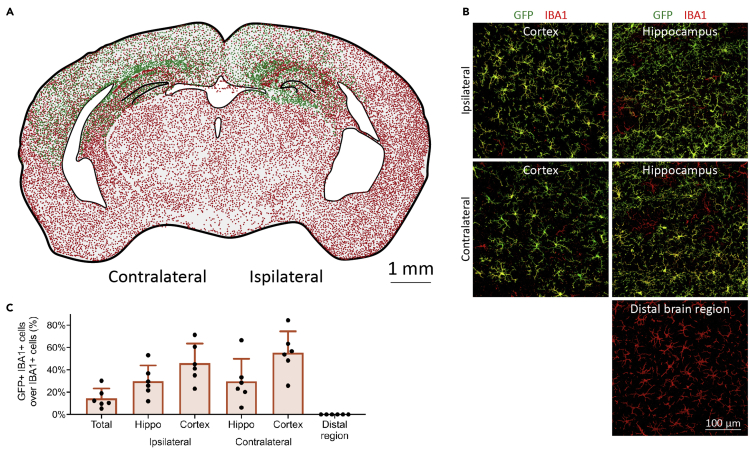
At D74, about half of IBA1-positive microglia are GFP-positive at the injected hippocampus and contralateral hippocampus (Figure 9), whereas the other brain regions were not obviously affected (Figure 9).
Figure 9.
Mr MT allows foreign microglia to locally replace endogenous microglia at the specified brain region
(A) Representative spatial distribution shows that Mr MT is able to achieve allogenic microglia replacement at the specified brain region. Each green dot represents a GFP-positive IBA1-positive engrafted microglial cell while each red dot represents a GFP-negative IBA1-positive endogenous microglial cell. Scale bar: 1 mm.
(B) Confocal images show that engrafted GFP-positive microglia repopulate the somatosensory cortex and hippocampus at both ipsilateral and contralateral injection hemispheres, whereas microglia in the distal region are not affected. Scale bar: 100 μm.
(C) Quantifications of Mr MT cell percentage in different brain regions. Mouse number: 6.
Red: IBA1; green: GFP. Data are presented as mean ± SD.
Figure reprinted with permission from Xu et al. (2020).
Limitations
Different from Mr BMT and microglia replacement by peripheral blood (Mr PB, original name mrPB) (Xu et al., 2020) (to editor: please also cite the Mr MT STAR Protocols papers STAR-PROTOCOLS-D-21-00164 and STAR-PROTOCOLS-D-21-00165 we submitted simultaneously), Mr MT does not achieve microglia replacement at the whole CNS scale. On the contrary, Mr MT can specifically replace endogenous microglia at specific brain region of interest. This may achieve specific microglia replacement upon specific purposes.
Mr BMT and Mr PB are noninvasive, whereas the procedure of Mr MT is invasive. Because Mr MT depends on intracerebral Injection, the injury and the following breakdown of the blood brain barrier may cause some side effect, such as inflammation, blood cell infiltration and competing with the transplanted microglia, and astrogliosis. The exact influence of these side effects on microglia replacement efficiency remains to be investigated.
The procedure of Mr MT is longer than Mr BMT and Mr PB. Because after endogenous microglia depletion, peripheral monocytes will infiltrate the brain and take up the emptied niche. To keep these niche vacant until donor microglia are transplanted, we have to modify the peripheral monocytes so that they can be eliminated via drug application while not affecting the transplanted microglia. In Mr MT, we exchanged the host peripheral monocytes to a cell type that expressed diphtheria toxin alpha subunit upon tamoxifen administration, so when tamoxifen is delivered to the host mouse, the peripheral monocytes will be eliminated and cannot compete with the transplanted microglia. The whole procedure is somewhat cumbersome. We will simplify the process in future.
Unilateral transplanted microglia can migrate to the contralateral brain region. In this case, we cannot control the distribution of transplanted microglia precisely as we intended. In scenarios where precise localization of transplanted microglia was preferred, Mr MT still needs futher refinement.
The microglia replacement is largely dependent on the microglia-free niche. In our hand, if the microglial depletion is not sufficient, the replacement efficiency could be low. Since the microglia are immune cells and sensitive to the microenvironment. An SPF animal facility is recommended. Otherwise, the microglial depletion and subsequent replacement efficiencies could be low.
Troubleshooting
Problem 1
Low efficiency of microglia replacement after Mr BMT procedure (step 9f in the paper Xu et al., 2021)
Potential solution
Check the efficiency of microglial depletion in Mr BMT at day 14 post PLX5622 administration. If the microglial depletion is not sufficient, check if the PLX5622 chemical is valid and the animal facility is SPF.
Check if the irradiation in Mr MBT procedure is conducted by X-ray. Gamma-ray usually results in much lower replacement efficiency.
Problem 2
Low efficiency of microglia depletion (steps 1–3 in the paper Xu et al. (2021)) .
Potential solution
In our experience, an SPF environment and chemically defined feeding chows are crucial for efficient depletion of microglia. Also, change the PLX5622 chow every 3 days in case the drug decomposes at the room temperature. Following these rules, the efficiency of microglia depletion can achieve as high as 99% after 14 days of PLX5622 treatment.
Problem 3
Failure of intravenous injection (step 9a-e in the paper Xu et al. (2021))
Potential solution
Using an injection cone equipped with LED light and a magnifier will be helpful to locate the tail vein. But the key to reliable success rate of intravenous injection via tail vein is constant practicing. Also keep in mind that never insert the needle’s full bevel into the tail, since the vein’s location is very shallow
Problem 4
A high death rate of mice after irradiation (steps 4–7 in the paper Xu et al. (2021))
Potential solution
Before irradiation, make sure that the operator’s tail vein injection technique has a success rate near 95%, or it would not in the animals’ welfare. Also, after irradiation, the mice should be given acidified drink water (pH 2–3) containing antibiotics for 2 weeks.
Problem 5
Low yield of donor microglia (step 34 in this paper).
Potential solution
The most likely reason is that the enzyme digestion and trituration process went wrong. Check if cysteine is added, since it was needed for papain activity. Also verify that the digestion temperature is ∼35°C, or the 20 min digestion won’t be enough. Finally, if lots of large pieces of brain tissues (>2 mm in diameter) were observed after papain neutralization, do not triturate the digested brain pieces with regular pipette tips when the pieces’ size is more than 2 mm, since the cells dissociated in this manner will not be healthy. Use tips that have been enlarged (for the first round of trituration) to triturate the brain pieces, till no pieces were large than 1 mm in diameter.
Problem 6
Low efficiency of microglia replacement (step 49 in this paper).
Potential solution
Ensure that bone marrow transplantation operator has near 95% success rate. Also ensure that the mice feed on tamoxifen chow. Change the tamoxifen chow every 3 days in case tamoxifen decomposes at room temperature.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Bo Peng (bopeng@connect.hku.hk).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The data that support the findings of this study are available from the corresponding author Bo Peng at Fudan University for reasonable request.
Acknowledgments
The authors thank Fang Lei (Fudan University) for the excellent laboratory management. The authors show their gratitude and respect to all animals sacrificed in this study. This study was supported by the National Key R&D Program of China (grant no. 2017YFC0111202) (B.P.), National Natural Science Foundation of China (grant no. 31922027) (B.P.), (grant no. 32000678) (Y.R.), and 31800871 (Z.X.), the Innovative Research Team of High-Level Local University in Shanghai (B.P.), Shenzhen Science and Technology Research Program (grant no. JCYJ20180507182033219) (B.P.), and grants of Chinese Academy of Sciences (172644KYSB20190077).
Author contributions
B.P. and Y.R. conceived, designed, and conceptualized this study. B.P. supervised this study. Z.X., B.P., and Y.R. wrote the manuscript. Z.X. performed most of experiments. B.P. and Y.R. contributed to the interpretation of results. All authors discussed results and commented on the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100665.
Contributor Information
Bo Peng, Email: bopeng@connect.hku.hk.
Yanxia Rao, Email: yanxiarao@connect.hku.hk.
References
- Xu Z., Rao Y., Huang Y., Zhou T., Feng R., Xiong S., Yuan T.F., Qin S., Lu Y., Zhou X. Efficient strategies for microglia replacement in the central nervous system. Cell Rep. 2020;32:108041. doi: 10.1016/j.celrep.2020.108041. [DOI] [PubMed] [Google Scholar]
- Xu Z., Zhou X., Peng B., Rao Y. Microglia replacement by bone marrow transplantation (Mr BMT) in the central nervous system of adult mice. STAR Protocols. 2021 doi: 10.1016/j.xpro.2021.100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author Bo Peng at Fudan University for reasonable request.