Figure 1 .
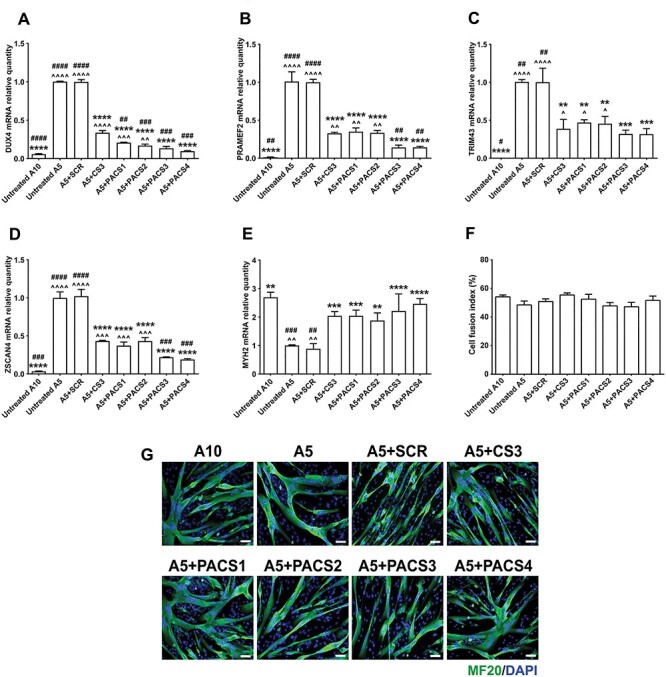
PMOs efficiently inhibit expression of DUX4 and downstream targets in immortalized FSHD myoblast cell cultures. Immortalized A5 myoblasts were differentiated for 2 days before the cells were treated with 10 μm PMOs through Endo-Porter–mediated transfection. Immortalized A10 or A5 cells receiving only Endo-Porter reagent were considered as untreated positive or negative control, respectively. Total RNA was extracted two days after PMO treatment. RT-qPCR quantification for DUX4 (A) and its targets: PRAMEF2 (B), TRIM43 (C), ZSCAN4 (D) and a marker of cell differentiation, MYH2 (E), are shown, relative to corresponding B2M expression. Cells in a parallel study were immunostained for all myosin isoforms using MF20 antibody. Cell fusion indexes were evaluated as the number of nuclei in MF20-positive myotubes containing ≥3 nuclei and expressed as the percentage of the total nuclei number in the image field (F). Representative cell images are displayed at magnification of ×100, scale bar = 100 μm, MF20 (green), DAPI (blue) (G). Statistical comparison (A–F) was by one-way ANOVA, followed by Tukey’s multiple comparisons test. Carets, asterisks or hashes indicate significances compared with untreated A10, untreated A5 or A5 treated with PMO CS3 (considered as positive PMO control), respectively. Data are shown as mean ± SEM, n = 3, P < 0.05 (*, ^, #), P < 0.01 (**, ^^, ##), P < 0.001 (***, ^^^, ###), P < 0.0001 (****, ^^^^, ####).
