Abstract
Background/aim
The prevalence of Helicobacter pylori is reported to be roughly 80% in Turkey, and only very few culture-based studies are available on antibacterial resistance in adult dyspeptic patients. This study was carried out in adult dyspeptic patients with an aim to: (i) detect H. pylori by invasive tests (culture, polymerase chain reaction, and histopathology) and (ii) determine the current resistance rates of H. pylori isolates to six antibiotics, including rifampicin.
Materials and methods
This study was conducted in 422 adult dyspeptic patients. The presence of H. pylori was demonstrated by culture, polymerase chain reaction, and the histopathology of gastric biopsy material. Antibacterial susceptibility was determined with the E-test.
Results
The mean age of the patients was 50 ± 15 (range 18–90), and 265 (63%) of them were female. By culture, polymerase chain reaction, and histopathology, the presence of H. pylori was detected at rates of 35% (148/422), 67% (281/422), and 53% (224/422), respectively. The prevalence of H. pylori was determined as 75.6% (319/422). Metronidazole, levofloxacin, clarithromycin, and rifampicin resistance rates were 62%, 36%, 19%, and 12%, respectively. Monodrug, dual-drug, and multidrug resistance rates were ascertained as 36.9%, 29.4%, and 10.5%, respectively. All of the isolates were susceptible to amoxicillin and tetracycline.
Conclusion
This study revealed the current prevalence of H. pylori in adult dyspeptic patients as 75.6%, and thereby, showed that infection with this pathogen remains highly prevalent. Although resistance to metronidazole and levofloxacin has increased over time, clarithromycin resistance rate has decreased. The high levels of resistance to metronidazole and levofloxacin limit the empirical use of these antibiotics in the eradication protocol. Owing to the low level of resistance determined for rifampicin, this antibiotic could be included in the eradication protocol, in the event of the need for rescue therapy in Turkey.
Keywords: Antibacterial resistance, clarithromycin, Helicobacter pylori, invasive test, rifampicin, Turkey
1. Introduction
Helicobacter pylori is a gram-negative microaerophilic bacterium acquired orally during childhood, and is known to colonize the gastric mucosa [1,2]. Being estimated to have colonized almost half of the world population, this pathogen may cause gastritis, peptic ulcer, gastric malignancies, immune thrombocytopenic purpura, iron deficiency anaemia, and vitamin B12 deficiency. Thus, H.pylori is considered a major public health concern [3–6]. The Maastricht IV and V/Florence Consensus Reports suggest that, to succeed in managing the diseases mentioned above, the presence of H. pylori s hould be investigated and eradication treatment should be instituted [4,6]. Furthermore, the Kyoto Global Consensus Report highlights that H. pylori eradication could prevent the aforementioned diseases, and also describes gastric colonisation with this bacterium as an infectious disease, irrespective of the clinical signs and symptoms observed [7].
H. pylori can be detected by several invasive methods, including culture, polymerase chain reaction (PCR), the rapid urease test, and histopathological examination, or by noninvasive methods such as the H. pylori s tool antigen test, urea breath test (UBT), and serological tests [8–11].
To date, a global standard treatment protocol has not been established for H. pylori . Therefore, treatment is based on the combined administration of empirically selected two or three antibiotics and the use of high doses of proton-pump inhibitors (PPIs). However, the success rate of this empirical treatment remains unsatisfactory due to increased levels of multidrug resistance [12].
As resistance to the antibiotics used for the eradication of H. pylori is increasing at a global level and eventually hinders treatment, it is suggested that the up-to-date susceptibility status of these antibiotics be determined by culture and an eradication programme be developed accordingly in each country [13].
In Bursa province, where the present study was conducted, the preceding investigation of the antibiotic resistance of H. pylori isolates was performed 12 years ago [14]. To the authors’ knowledge, there is no investigation on the antibiotic resistance status of H. pylori in Bursa, other than the mentioned study [14]. In addition, there is no study on rifampicin (RD) resistance in H. pylori isolates from Turkey.
This study was carried out in adult dyspeptic patients with an aim to: (i) detect H. pylori by invasive tests (culture, polymerase chain reaction, and histopathology), and (ii) determine the current resistance rates of H. pylori isolates to six antibiotics, including rifampicin.
2. Material and methods
2.1. Patients
In total 422 adult dyspeptic patients were enrolled in this cross-sectional study. These patients underwent upper gastrointestinal (GI) endoscopy under sedation at the Department of Gastroenterology, Bursa Yüksek İhtisas Training and Research Hospital, University of Health Sciences, Turkey, from March 2018 to September 2019. The adult patients included in this study were aged 18 and over, presented with dyspeptic symptoms (postprandial fullness, early satiation, epigastric pain, and epigastric burning) and were referred for upper GI endoscopy. Patients, known to be pregnant or diagnosed with coagulation disorders and to have received prior eradication treatment for H. pylori or undergone gastric surgery, were excluded from the study. Patients with a medical history of antibiotic use in the last 6 weeks were also excluded. Demographic and endoscopic data were recorded for all patients. All participants provided written informed consent before participating in the study. This study was approved by the Local Research Ethics Committee of Erciyes University Medical Faculty and designed in accordance with the 2013 Brazil version of the Helsinki Declaration. The Ethics Committee reference number is 2018/135.
2.2. Gastric biopsy specimens
The upper GI endoscopy procedure involved the collection of four gastric biopsy specimens from each patient, including two from the antrum and two from the corpus. Two of the specimens (1 antrum, 1 corpus) were transferred into 10% formalin solution and used for histopathological examination. The other two specimens were transferred into sterile Eppendorf tubes, containing 500 µL of brain heart infusion (BHI) broth (CM1135B, Thermo Fisher Scientific, Waltham, MA, USA), for bacteriological and molecular analyses. Histopathological examination was performed at the Department of Pathology, Bursa Yüksek İhtisas Training and Research Hospital, University of Health Sciences. Bacteriological and molecular analyses were conducted at the Department of Microbiology, Faculty of Veterinary Medicine, Erciyes University.
2.3. Histopathological examination
The biopsyspecimens were fixed in 10% formalin solution and embedded in paraffin. The sections cut from the paraffin blocks were stained with haematoxylin and eosin (H&E) and examined by an experienced pathologist [15].
2.4. Culture
The biopsy specimens maintained in BHI broth (CM1135B, Thermo Fisher Scientific) were ground using a sterile glass rod and homogenized. Twenty microliters of this material was inoculated onto Columbia blood agar base (CM0331B, Thermo Fisher Scientific) enriched with 10% defibrinated horse blood and Dent supplement (SR0147E, Thermo Fisher Scientific). The inoculated plates were incubated at 37 °C in a microaerobic atmosphere (Anaerocult C, Merck Millipore, Darmstadt, Germany) for 7–10 days. At the end of the incubation period, the H. pylori -suspect colonies (S type, gram-negative, and oxidase-, catalase-, and urease-positive) were assessed and their pure cultures were prepared [16,17]. The pure cultures of the isolates were stored in BHI broth supplemented with 15% glycerol at –84 °C for further use in molecular analyses and antibacterial susceptibility tests.
2.5. Molecular analysis
a) DNA extraction
Commercial microbial DNA isolation kits were used to extract DNA from both the biopsy samples (Roche high pure PCR Template Preparation Kit, Mannheim, Germany) and the H. pylori isolates obtained from culture (DNeasy UltraClean Microbial Kit, Qiagen, Hilden, Germany). The extraction procedures were performed in accordance with the manufacturer’s instructions.
b) Polymerase chain reaction (PCR)
The PCR method was used to demonstrate the presence of H. pylori in the gastric biopsy specimens and to confirm the identification of the isolates recovered from the selective agar as H. pylori by phenotypic tests. For this purpose, primers (glmM-F 5’-AAGCTTTTAGGGGTGTTAGGGGTTT-3’ and glmM-R 5’-AAGCTTACTTTCTAACACTAAC GC-3’) specific to the phosphoglucosamine mutase (glmM) gene of H. pylori were used, and the test was performed by a method of Lu et al. [18]. The amplification cycles consisted of denaturation at 93 °C for 1 min, primer annealing at 58 °C for 1 min, and extension at 72 °C for 1 min. Samples were amplified through 35 cycles. Amplified products were resolved by gel electrophoresis on 1.5% agarose (Biomax, Agarose, Lot no. 124543PR, from Prona, European Economic Community) and visualized under a UV transilluminator (G:BOX Chemi XRQ; Syngene, Cambridge, UK). Bands, which were 294-bp in size, were considered as a positive result.
2.6. Antibacterial susceptibility testing
The minimal inhibitory concentration (MIC) values of amoxicillin, clarithromycin, levofloxacin, metronidazole, rifampicin, and tetracycline against the H. pylori isolates were determined with the E-test. Mueller–Hinton agar supplemented with 7% defibrinated horse blood (R54092, Thermo Fisher Scientific) was used for this purpose. Accordingly, a suspension prepared from 3-day-old H. pylori cultures (Mac Farland No:3) was spread onto Mueller–Hinton agar (CM0337B, Thermo Fisher Scientific) supplemented with 7% defibrinated horse blood, and after the agar surface dried, the E-test (The Liofilchem MIC Test Strips, Italy) strips of the selected antibiotics were placed onto the agar. The plates were incubated at 37 °C in a microaerobic environment for 48–72 h [16]. The results were evaluated according to the criteria of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [19]. The MIC values (mg/L) of amoxicillin, clarithromycin, tetracycline, metronidazole, levofloxacin, and rifampicin were≤0.125, ≤0.5, ≤1, ≤8, ≤1, and ≤1, respectively. Isolates, which were resistant to three or more antibiotics, were considered to be multidrug resistant.
2.7. Standard strain
Helicobacter pylori ATCC 700824 was used as a positive control in culture, molecular analysis, and antibiotic susceptibility tests.
2.8. Criterion used to detect the presence of H. pylori
H. pylori was considered to be present in the samples, when the result of any of the invasive tests (culture, PCR, and histopathology) was positive.
2.9. Statistical analysis
Data were analysed using the SPSSversion 20.0 (IBM Corporation, Armonk, NY, USA). Values were expressed as mean ± standard deviation for normally distributed variables, and as count and percent for categorical variables.
3. Results
3.1. Clinical data
In total, 422 adult dyspeptic patients were included in this study. The mean age of the patients was 50 ± 15 years (range: 18–90) and 265 (63%) of them were female. The most common endoscopic finding was gastritis, accounting for 62%, followed by reflux esophagitis (16%), duodenal ulcer/duodenitis (15%), benign gastric ulcer (4.5%), normal gastric mucosa (2%), and gastric malignancy (0.5%) (Table 1). Gastritis, benign gastric ulcer, normal gastric mucosa, and gastric malignancy were also demonstrated histopathologically.
Table 1.
Demographic and clinical data of the patients (n: 422).
| Characteristics | n (%) | |
|---|---|---|
| Age (mean ± SD) | 50 ± 15 | |
| Sex | ||
| Male | 157 (37) | |
| Female | 265 (63) | |
| Endoscopic findings | ||
| Gastritis | 261 (62) | |
| Reflux esophagitis | 68 (16) | |
| Duodenal ulcer/duodenitis | 62 (15) | |
| Gastriculcer | 19 (4.5) | |
| Normal | 10 (2) | |
| Gastric malignancy | 2 (0.5) | |
SD: Standard deviation.
3.2. Prevalence of H. pylori and results of the invasive tests
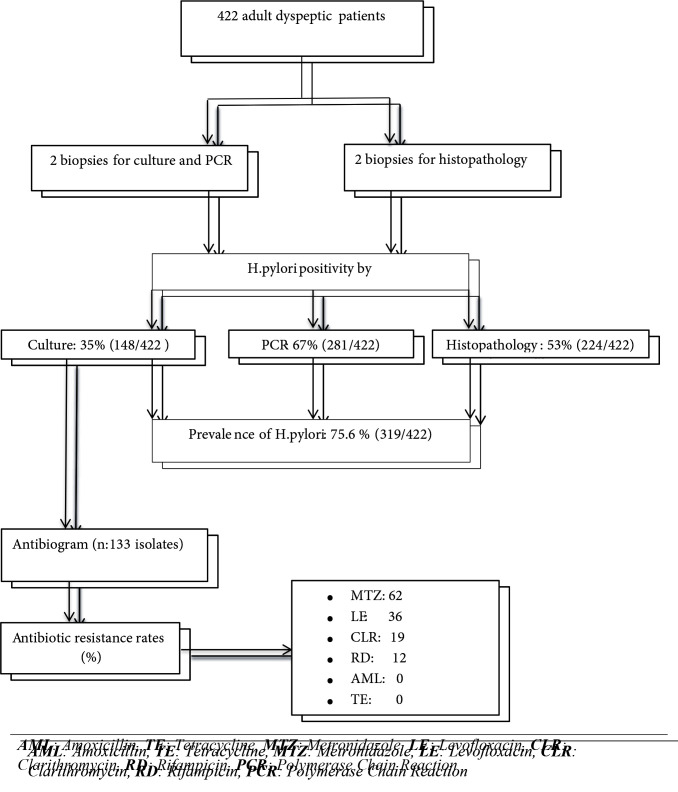
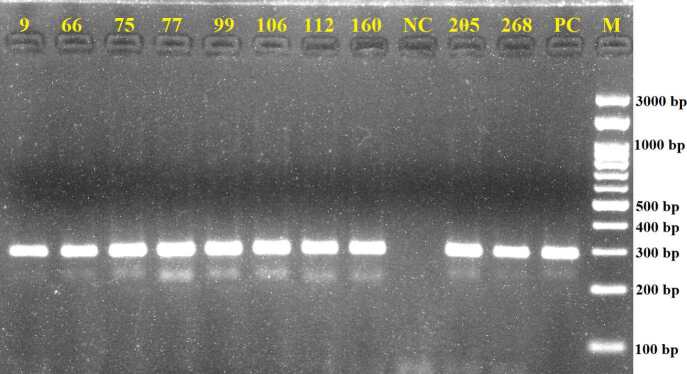
The prevalence of H. pylori was determined as 75.6% (319/422). The presence of H. pylori was detected at rates of 35% (148/422), 67% (281/422), and 53% (224/422) by culture, PCR, and histopathological examination, respectively (Figure 1). A representative image of the agarose gel electrophoresis of the PCR products is shown in Figure 2.
Figure 1.
Flow diagram of the current study.
Figure 2.
Agarose gel electrophoresis of PCR products obtained by using primer pairs glmM-F and glmM-R (297bp). NC: Negative control (distilled water), PC: Positive control (H. pylori, ATCC 700824), Representative positive samples (9, 66, 75, 77, 99, 106, 112, 160, 205, 268): H. pylori isolates recovered from gastric biopsies in the current study M: Marker, 100bp DNA Ladder H3 RTU, GeneDireX.
3.3. Antibacterial susceptibility testing
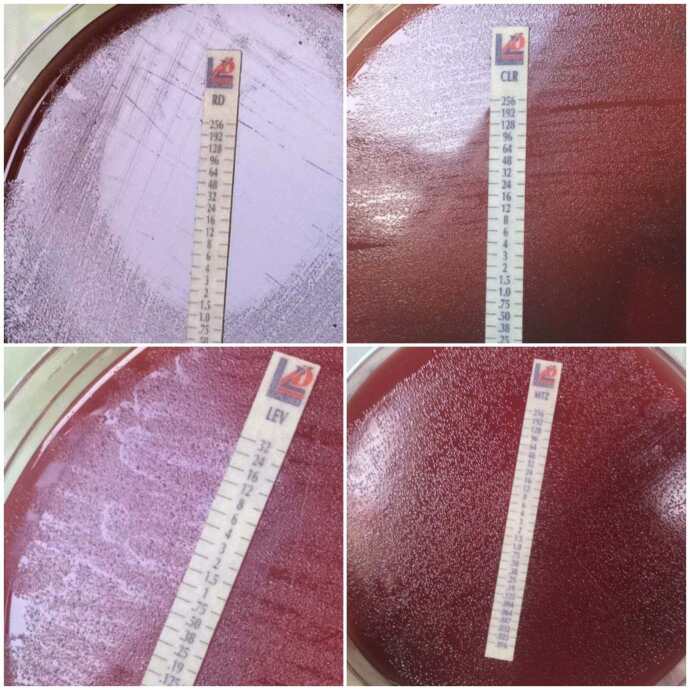
Out of the 148 H. pylori isolates recovered from culture, 15 could not be subcultured with passages. Thus, 133 isolates were tested for antibacterial susceptibility. Based on the test results, all 133 (100%) isolates were susceptible to amoxicillin and tetracycline. Metronidazole, levofloxacin, clarithromycin, and rifampicin resistance were determined at levels of 62%, 36%, 19%, and 12%, respectively (Figure 1). A representative image of the E-test is shown in Figure 3. A detailed assessment of the test results demonstrated that, out of the 133 isolates, 31 (23.3%) were susceptible to all of the antibiotics tested, 49 (36.9%) were resistant to a single antibiotic, 39 (29.4%) were resistant to two antibiotics, and 14 (10.5%) were multidrug resistant (Table 2).
Figure 3.
Representative images regarding E-test rifampicin, clarithromycin, levofloxacin, and metronidazole.
Table 2.
Antibiotic susceptibility profiles of the H. pylori isolates (n: 133).
| Profiles | n (%) | |
|---|---|---|
| No resistance to all drugs | 31 (23.3) | |
| Monodrug resistance | 49 (36.9) | |
| MTZ LE CLR RD | 37 (27.8)9 (6.8)1 (0.8)2 (1.5) | |
| Dual-drugs resistance | 39 (29.4) | |
| MTZ+LEMTZ+CLRMTZ+RDLE+CLRLE+RD | 21 (15.8)6 (4.5)6 (4.5)5 (3.8)1 (0.8) | |
| Multidrugs resistance | 14 (10.5) | |
| Triple resistance | 12 (9.1) | |
| MTZ+LE+CLRMTZ+LE+RDMTZ+CLR+RDLE+CLR+RD | 7 (5.3)1 (0.8)2 (1.5)2 (1.5) | |
| Quadruple resistance | 2 (1.5) | |
| MTZ+LE+CLR+RD | 2 (1.5) | |
MTZ: Metronidazole, LE: Levofloxacin, CLR: Clarithromycin, RD: Rifampicin.
4. Discussion
To the authors’ knowledge, the present study is the most comprehensive molecular and culture-based research conducted to date on H.pylori in Turkey. Furthermore, this study is the first to report rifampicin resistance in H.pylori isolates from Turkey.
The prevalence of H. pylori varies greatly in developed and developing countries (18.9–87.7%) [20]. In a community-based survey conducted in 4663 adults in Turkey, the prevalence of H. pylori was determined as 82.5% with the UBT [21]. The prevalence of H. pylori in dyspeptic patients is reported to range between 17% and 34% in developed countries [22,23], 75.6% and 86% in developing countries [24,25], and 59.6% and 71.3% in Turkey [26,27]. In the present study, the prevalence of H. pylori (75.6%) was higher than the prevalence reported for developed countries and similar to that reported for developing countries.
As is the case throughout the world, in Turkey, the antibiotics most commonly used for the eradication of H. pylori are clarithromycin, metronidazole, levofloxacin, tetracycline, and amoxicillin [28–31]. Rifampicin is generally used for the treatment of tuberculosis, therefore, in order to prevent the development of resistance, this antibiotic is purposefully not used for the empirical treatment of H. pylori in Turkey.
Reports have shown that the resistance of H. pylori to these six antibiotics varies among both countries and regions [12,13,32–35]. In most of the WHO regions, clarithromycin, metronidazole, and levofloxacin resistance rates of >15% have been reported [13,36]. Furthermore, tetracycline and amoxicillin resistance rates have been reported to be <10% across the globe [36,37]. It has also been reported that, rifampicin resistance ranges between 0% and 33% [16,36,38–40]. In addition, clarithromycin, metronidazole, levofloxacin, tetracycline, and amoxicillin resistance rates range between 18% and 34%, 28.7% and 56%, 0% and 22%, 0% and 10%, and 0% and 14%, respectively, in different regions of the world [13,16,39–41], and are 3.6% and 28.1%, 33.8% and 64.9%, 8% and 21.9%, 16.1%, and 20.7%, respectively, in the neighbouring countries of Turkey [38,40,42,43].
According to research conducted in different regions of Turkey in the past decade, clarithromycin, metronidazole, levofloxacin, tetracycline, and amoxicillin resistance rates range between 18.1% and 38.1%, 31.1% and 45.5%, 18.2% and 34%, 0% and 9.1%, and 0% and 2.9%, respectively [27,44–46]. In a previous study carried out in Bursa province, which is also the location of the present study, the rates of clarithromycin, metronidazole, tetracycline, and amoxicillin resistance were found to be 41.9%, 41.9%, 3.2%, and 3.2%, respectively [14] (Table 3).
Table 3.
H. pylori antibiotic resistance rates among adults in recent years in different regions of Turkey.
| Study year | Method | Number of patients | Number of isolates | Region(city) | Resistance rate (%) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AML | TE | MTZ | CLR | LE | RD | |||||||
| 2008 | E-test | 31 | 31 | West (Bursa) | 3.2 | 3.2 | 41.9 | 41.9 | - | - | 14 | |
| 2010 | E-test | 149 | 102 | South (Mersin) | 0 | 9.1 | 45.5 | 18.1 | 18.2 | - | 46 | |
| 2011 | E-test | 344 | 104 | North (Trabzon) | 2.9 | 1 | 31.1 | 28.2 | 34 | - | 27 | |
| 2013 | E-test | 98 | 98 | West (İstanbul) | 0 | 0 | 35.5 | 36.7 | 29.5 | - | 44 | |
| 2017 | E-test | 63 | 63 | West (İstanbul) | - | - | - | 38.1 | - | - | 45 | |
| This study | E-test | 422 | 133 | West (Bursa) | 0 | 0 | 62 | 19 | 36 | 12 | - | |
AML: Amoxicillin, TE: Tetracycline, MTZ: Metronidazole, LE: Levofloxacin, CLR: Clarithromycin, RD: Rifampicin, Ref.: Reference.
The present study demonstrated that clarithromycin resistance (19%) had decreased over time in Turkey, and this was attributed to the clinical use of clarithromycin having decreased. On the other hand, metronidazole (62%) and levofloxacin (36%) resistance were ascertained to have increased in Turkey. This could be attributed to the increased clinical use of this antibiotic as well as to patients not adhering to the prescribed administration dose and period. In view of the high levels of clarithromycin, metronidazole, and levofloxacin resistance detected in Turkey, it is suggested that the empirical use of these antibiotics for the eradication of H. pylori should be avoided, and their clinical use should be based on antibacterial susceptibility test results. In addition, we determined that all of the tested H. pylori isolates were susceptible to tetracycline and amoxicillin. Therefore, it is considered that both tetracycline and amoxicillin can be safely used for the empirical treatment of H. pylori in Turkey. To the authors’ knowledge, rifampicin resistance has not been investigated before in H. pylori isolates from Turkey. The present study reports, for the first time, the resistance level of H. pylori to rifampicin as 12%. The level of resistance to rifampicin is lower than the resistance levels to the antibiotics used for the eradication of H. pylori in Turkey, including clarithromycin, metronidazole, and levofloxacin. This result suggests that, rifampicin could be included in the eradication protocol of H. pylori , when salvage therapy is needed in Turkey.
Worldwide, the multidrug resistance rate of H. pylori is reported as 9.6% [37]. In the present study, the rate of resistance to at least one antibiotic was ascertained as 76.6% and multidrug resistance was determined at a rate of 10.5% (Table 2). Therefore, to achieve success in the eradication of H. pylori , it is required to determine the current antibiotic resistance status, and to establish appropriate eradication protocols in view of this status.
4.1. Limitations of the study
i-) The current study is monocentric, so the results obtained may not accurately represent the entire territory of Turkey.
ii-) This study does not include a sufficient number of patients to determine the exact prevalence of H. pylori in adult dyspeptic patients.
4. Conclusion
The prevalence of H. pylori in adult dyspeptic patients was found to be higher than that in developed countries and similar to that in developing countries. Resistance to metronidazole and levofloxacin was determined to have increased over time. Although resistance to clarithromycin had decreased over time, it was observed to remain above 15%. It is considered that the high levels of resistance detected in H. pylori isolates to metronidazole and levofloxacin would constitute a major problem in empirical treatment. On the other hand, as no resistance was detected to amoxicillin and tetracycline, these antibiotics were considered to be safe for use in the eradication of H. pylori. Furthermore, in view of the low level of resistance to rifampicin, this antibiotic could be included in the eradication protocol of H. pylori in Turkey as an option for rescue therapy.
Disclaimers/conflict of interest
The manuscript has not been published previously elsewhere. There is no specific funding has been received to report this submission. All of the authors declare that they have all participated in the design, execution, and analysis of the paper, and that they have approved the final version. All authors are in agreement with the content of the manuscript. The authors have no conflict of interest to disclose.
References
- Magalhães Queiroz DM Luzza F Epidemiology of Helicobacter pylori infection. Helicobacter . 2006;11:1–5. doi: 10.1111/j.1478-405X.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- Xia HH Talley NJ Natural acquisition and spontaneouse limination of Helicobacter pylori infection: clinical implications. The American Journal of Gastroenterology . 1997;92:1780–1787. [PubMed] [Google Scholar]
- Beswick EJ Suarez G Reyes VE Helicobacter pylori and host interactions that influence pathogenesis. World Journal ofGastroenterology . 2006;12:5599–5605. doi: 10.3748/wjg.v12.i35.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfertheiner P Megraud F Morain O Atherton CA Axon J AT Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut . 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P Chan FK McColl KE Peptic ulcer disease. Lancet . 2009;374:1449–1461. doi: 10.1016/S0140-6736(09)60938-7. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P Megraud F Morain O Gisbert CA Kuipers JP EJ Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut . 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- Sugano K Tack J Kuipers EJ Graham DY El-Omar EM Kyoto global consensusreport on Helicobacter pylori gastritis. Gut . 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon BJ Bruce MG Koch A Goodman KJ Tsukanov V The diagnosis and treatment of Helicobacter pylori infection in Arctic regions with a high prevalence of infection: expert commentary. Epidemiology and Infection . 2016;144:225–233. doi: 10.1017/S0950268815001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YK Kuo FC Liu CJ Wu MC Shih HY Diagnosis of helicobacter pylori infection: current options and developments. World Journal of Gastroenterology . 2015;21:11221–11235. doi: 10.3748/wjg.v21.i40.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG Jung HK Lee HL Jang JY Lee H Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Journal of Gastroenterology and Hepatology . 2014;29:1371–1386. doi: 10.1111/jgh.12607. [DOI] [PubMed] [Google Scholar]
- Diagnosis of Helicobacter pylori using invasive and noninvasive approaches. Journal of Pathogens . 2018;10 doi: 10.1155/2018/9064952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan N Yılmaz Ö Demiray-Gürbüz E Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World Journal of Gastroenterology . 2017;23:2854–2869. doi: 10.3748/wjg.v23.i16.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldi A Carrara E Graham DY Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology . 2018;155:1372–1382. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakır Özbey S Özakın C Keskin M. Antibiotic resistance rates of Helicobacter pylori isolates and the comparison of E-test and fluorescent in situ hybridization methods for the detection of clarithromycin resistant strains. Mikrobiyoloji Bulteni . 2009;43:227–234. [PubMed] [Google Scholar]
- Benoit A Hoyeau N Fléjou JF Diagnosis of Helicobacter pylori infection on gastric biopsies: standard stain, special stain or immunohistochemistry? Annales de Pathologie . 2018;38:363–369. doi: 10.1016/j.annpat.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Macías-García F Llovo-Taboada J Díaz-López M Bastón-Rey I Domínguez-Muñoz JE High primary antibiotic resistance of Helicobacter Pylori strains isolated from dyspeptic patients: a prevalence cross-sectional study in Spain. Helicobacter . 2017;22 doi: 10.1111/hel.12440. [DOI] [PubMed] [Google Scholar]
- UK Standards for Microbiology Investigations. Bacteriology identification: 26 . 2015. pp. 1–27.
- Lu JJ Perng CL Shyu RY Chen CH Comparison of five PCR methods for detection of Helicobacter pylori DNA in gastrict issues. Journal of Clinical Microbiology . 1999;37:772–774. doi: 10.1128/jcm.37.3.772-774.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters Version 10. 2020;01:01–01. [Google Scholar]
- Hooi JKY Lai WY Ng WK Suen MMY Underwood FE Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology . 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Ozaydin N Turkyilmaz SA Cali S. Prevalence and risk factors of Helicobacter pylori in Turkey: a nationally-representative, cross-sectional, screening with the ¹³C-urea breath test. BMC Public Health . 2013;13:1215–1215. doi: 10.1186/1471-2458-13-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnelli G Cuomo R Janssens J Tack J Symptom patterns and pathophysiological mechanisms in dyspeptic patients with and without Helicobacter pylori. Digestive Diseases and Sciences . 2003;48:2229–2236. doi: 10.1023/b:ddas.0000007856.71462.6c. [DOI] [PubMed] [Google Scholar]
- Duggan AE Elliott CA Miller P Hawkey CJ Logan RF. Clinical trial: a randomized trial of early endoscopy, Helicobacter pylori testing and empirical therapy for the management of dyspepsia in primary care. Alimentary Pharmacology & Therapeutics . 2009;29:55–68. doi: 10.1111/j.1365-2036.2008.03852.x. [DOI] [PubMed] [Google Scholar]
- Hamrah MH Hamrah MS Hassan Hamrah M Kanda M Hamrah AE Prevalence of Helicobacter pylori infection in dyspeptic patients in Andkhoy Afghanistan. Asian Pacific Journal of Cancer Prevention . 2017;18:3123–3127. doi: 10.22034/APJCP.2017.18.11.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorji D Dendup T Malaty HM Wangchuk K Yangzom D Epidemiology of Helicobacter pylori in Bhutan: the role of environment and geographic location. Helicobacter . 2014;19:69–73. doi: 10.1111/hel.12088. [DOI] [PubMed] [Google Scholar]
- Ozdil K Sahin A Kahraman R Yuzbasioglu B Demirdag H Current prevalence of intestinal metaplasia and Helicobacter pylori infection in dyspeptic adult patients from Turkey. Hepatogastroenterology . 2010;57:1563–1566. [PubMed] [Google Scholar]
- Erkut M Uzun DY Kaklıkkaya N Fidan S Sociodemographic characteristics and clinical risk factors of Helicobacterpylori infection and antibiotic resistance in the Eastern Black Sea region of Turkey. Turkish Journal of Gastroenterology . 2020;31:221–233. doi: 10.5152/tjg.2020.18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekilli M Onal IK Ocal S Dogan Z Tanoglu A Inefficacy of triple therapy and comparison of two different bismuth-containing quadruple regimens as a first line treatment option for Helicobacter pylori. Saudi Journal of Gastroenterology . 2016;22:366–369. doi: 10.4103/1319-3767.191141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M Tanoglu A Duzenli T Tozun AN Helicobacter pylori treatment in Turkey: Current status and rational treatment options. Nothern Clinics of İstanbul . 2019;7:87–94. doi: 10.14744/nci.2019.62558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin O Aydın MK Özdemir AA Kanık AE Standard tripletherapy in Helicobacter pylori eradication in Turkey: systematic evaluation and meta-analysis of 10-year studies. Turkish Journal of Gastroenterology . 2019;30:420–435. doi: 10.5152/tjg.2019.18693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ’Connor O Liou A Gisbert JM JP ’Morain C. O Review: treatment of Helicobacter pylori infection 2019. Helicobacter . 2019;24 doi: 10.1111/hel.12640. [DOI] [PubMed] [Google Scholar]
- Smith SM ’Morain O McNamara D C Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World Journal of Gastroenterology . 2014;20:9912–9921. doi: 10.3748/wjg.v20.i29.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota G Ianiro G Bibbò S DiRienzo TA Masucci L Culture-guided treatment approach for Helicobacter pylori infection: review of the literature. World Journal of Gastroenterology . 2014;20:5205–5211. doi: 10.3748/wjg.v20.i18.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ’connor O Taneike A Nami I Fitzgerald A Murphy N Helicobacterpylori resistance to metronidazole and clarithromycin in Ireland. European Journal of Gastroenterology & Hepatology . 2010;22:1123–1127. doi: 10.1097/MEG.0b013e328338e43d. [DOI] [PubMed] [Google Scholar]
- López-Góngora S Puig I Calvet X Villoria A Baylina M Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. Journal of Antimicrobial Chemotherapy . 2015;70:2447–2455. doi: 10.1093/jac/dkv155. [DOI] [PubMed] [Google Scholar]
- European survey of Helicobacter pylori primary resistance to antibiotics: evolution over the last 20 years. In: 30th European Congress on Clinical Microbiology and Infectious Diseases . 2020. pp. 10000–10000.
- De Francesco V Giorgio F Hassan C Manes G Worldwide Helicobacter pylori antibiotic resistance: a systematic review. Journal of Gastrointestinal and Liver Diseases . 2010;19:409–414. [PubMed] [Google Scholar]
- Khademi F Sahebkar A An updated systematic review and meta-analysis on the Helicobacter pylori antibiotic resistance in Iran ( Microbial Drug Resistance . 2010;10:2020–2020. doi: 10.1089/mdr.2020.0088. [DOI] [PubMed] [Google Scholar]
- Regnath T Raecke O Enninger A Ignatius R Increasing metronidazole and rifampicin resistance of Helicobacter pylori isolates obtained from children and adolescents between 2002 and 2015 in southwest Germany. Helicobacter . 2017;22 doi: 10.1111/hel.12327. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez B Georgopoulos S Michopoulos S Karayiannis Y Liatsos C Multicenter survey of antimicrobial resistance in Helicobacter pylori isolates in Greece—Trends of resistance 1998–2018. XXXIInd Int. Work. Helicobacter Microbiota Inflamm. Cancer . 2019;24:e12647–e12647. [Google Scholar]
- Kuo YT El-Omar EM Wu JY Leow AHR Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterology and Hepatology . 2017;2:707–715. doi: 10.1016/S2468-1253(17)30219-4. [DOI] [PubMed] [Google Scholar]
- Gemilyan M Hakobyan G Benejat L Allushi B Melik-Nubaryan D Prevalence of Helicobacter pylori infection and antibiotic resistance profile in Armenia. Gut Pathogens . 2019;11:28–28. doi: 10.1186/s13099-019-0310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyanova L Gergova G Evstatiev I Spassova Z Kandilarov N Helicobacter pylori resistance to six antibiotics by two breakpoint systems and resistance evolution in Bulgaria. Infectious Diseases . 2016;48:56–62. doi: 10.3109/23744235.2015.1082035. [DOI] [PubMed] [Google Scholar]
- Caliskan R Tokman HB Erzin Y Saribas S Antimicrobial resistance of Helicobacter pylori strains to five antibiotics, including levofloxacin. in Northwestern Turkey. Revista da Sociedade Brasileira de Medicina Tropical . 2015;48:278–284. doi: 10.1590/0037-8682-0027-2015. [DOI] [PubMed] [Google Scholar]
- Kocazeybek B Sakli MK Yuksel P Demirci M Comparison of new and classical point mutations associated with clarithromycin resistance in Helicobacter pylori strains isolated from dyspeptic patients and their effects on phenotypic clarithromycin resistance. Journal of Medical Microbiology . 2019;68:566–573. doi: 10.1099/jmm.0.000944. [DOI] [PubMed] [Google Scholar]
- Çağdaş U Otağ F Tezcan S Sezgin O Aslan G Detection of Helicobacter pylori and antimicrobial resistance in gastric biopsy specimens. Mikrobiyoloji Bülteni . 2012;46:398–409. [PubMed] [Google Scholar]