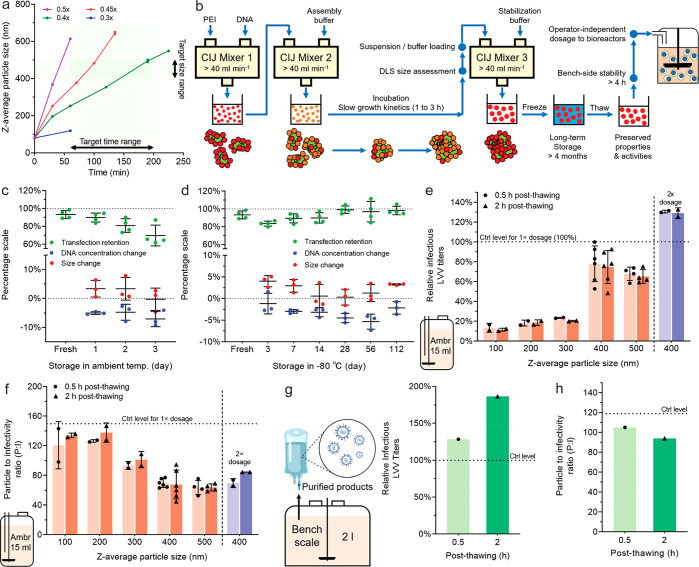
Figure 5.
Scale-up production of pDNA/PEI particles with controlled sizes and validation of transfection efficiency for LVV production in bioreactors. (a) Tunable particle size growth kinetics as a function of ionic strength of the particle growth medium (i.e., PBS concentration, 0.3×, 0.4×, 0.45×, and 0.5× of the full ionic strength). (b) Schematic of the scale-up production process enabled by conducting the mixing steps in CIJ mixers at a flow rate of higher than 40 mL/min. (c) Stability of the 400 nm particles at ambient temperature. (d) Stability of the 400 nm particles at different time points during storage at −80 °C. Particle suspension samples were thawed at ambient temperature before testing. (e, f) Effect of pDNA/PEI particle size on the infectious titers (e) and P:I ratios (f) of the LVVs produced in the 15 mL small bioreactors (ambr 15). A 1× dosage level represents 1 μg of pDNA/mL in the suspension cultures. The data power of each size group differed in the experimental design and is fully indicated by the individual data points shown in the figures. (g, h) The infectious titers (g) and P:I ratios (h) of LVVs produced in a 2 L bioreactor using the 400 nm pDNA/PEI particles, at a dosage level of 1 μg of pDNA/mL. n = 1 bioreactor for each condition. In (e–h), the control level represents the optimal results from the standardized in-house procedures to prepare pDNA/PEI particles manually immediately before the transfection experiments.