Abstract
Background and aims:
Small fiber neuropathy (SFN) is increasingly suspected in patients with pain of uncertain origin, and making the diagnosis remains a challenge lacking a diagnostic gold standard.
Methods:
In this case–control study, we prospectively recruited 86 patients with a medical history and clinical phenotype suggestive of SFN. Patients underwent neurological examination, quantitative sensory testing (QST), and distal and proximal skin punch biopsy, and were tested for pain-associated gene loci. Fifty-five of these patients additionally underwent pain-related evoked potentials (PREP), corneal confocal microscopy (CCM), and a quantitative sudomotor axon reflex test (QSART).
Results:
Abnormal distal intraepidermal nerve fiber density (IENFD) (60/86, 70%) and neurological examination (53/86, 62%) most frequently reflected small fiber disease. Adding CCM and/or PREP further increased the number of patients with small fiber impairment to 47/55 (85%). Genetic testing revealed potentially pathogenic gene variants in 14/86 (16%) index patients. QST, QSART, and proximal IENFD were of lower impact.
Conclusion:
We propose to diagnose SFN primarily based on the results of neurological examination and distal IENFD, with more detailed phenotyping in specialized centers.
Keywords: algorithm, diagnosis, neurological examination, skin punch biopsy, small fiber neuropathy
Introduction
Small fiber neuropathies (SFNs) form a subgroup of painful sensory neuropathies with dysfunction or damage of the thinly myelinated A-delta and unmyelinated C nerve fibers. 1 A diagnosis of SFN is increasingly suspected in patients with pain of uncertain origin. However, there is no diagnostic gold standard for SFN, and rigorous studies assessing the validity of the different small fiber tests are scarce.2–6 Current clinical practice relies on quantitative sensory testing (QST) and distal intraepidermal nerve fiber density (IENFD).4,5,7–9 The evidence for the additional benefit of novel functional, electrophysiological, and morphological small fiber tests and genetic testing for SFN was considered to be limited. Indeed, a recent systematic review has identified considerable heterogeneity in diagnostic criteria and highlighted the need for evidence based guidelines for the diagnosis of SFN. 6
We now report on a deep phenotyping and genotyping study in a cohort of unselected patients admitted to our neuromuscular center with suspected SFN, where we evaluated the utility of six small fiber tests and genetic testing. We hypothesized that adding novel small fiber tests to the clinical algorithm will increase the number of patients identified with small fiber pathology.
Patients and methods
Subjects and baseline characterization
Between 2015 and 2019, we recruited 86 adult patients (51 women, 35 men; median age 53 years, 19–78) who were admitted to the Department of Neurology, University of Würzburg, Germany with a suspected diagnosis of painful SFN. Patients were first seen as regular in- or out-patients. They were enrolled in our study if their symptoms were indicative of SFN (i.e. acral, focal and/or widespread pain and/or par-/dysesthesias, symptoms of autonomic dysfunction) and if neurological examination and nerve conduction studies did not reveal polyneuropathy or any other neurological disease potentially underlying the symptoms. Patients were excluded if they reported or had a documented diagnosis of diabetes mellitus, renal insufficiency, uncontrolled thyroid dysfunction, hypovitaminosis B12, acute infection, and malignancy within the last five years. Upon enrollment, patients additionally underwent the respective laboratory tests (see below). Some small fiber tests (see below) led to additional exclusion criteria such as epilepsy, eye diseases or surgery, usage of hard contact lenses, and cardiac pacemaker. We also excluded patients with pending compensation claims.
Patients underwent a complete neurological examination of all systems and additional specific tests for small fiber abnormality. This included testing for thermal/mechanical/pinprick hypoesthesia and hyperesthesia, hypoalgesia and hyperalgesia, touch paresthesia, and static and dynamic allodynia. Detailed sensory examination was performed at the extremities, that is, hands and feet, lower and upper legs, and lower and upper arms. We used the term hyperesthesia if a sensory stimulus, such as touch, was reported to feel more intense without being painful, and we used the term hyperalgesia if a normally painful stimulus (pinprick) was reported to be more painful. Neurological examination was considered indicative of small fiber dysfunction if one of these was abnormal. Patients were asked for autonomic symptoms, that is, sweating, orthostatic hypotension, and gastrointestinal distress, and completed the Neuropathic Pain Symptom Inventory,10,11 Graded Chronic Pain Scale, 12 Pain Catastrophizing Scale, 13 and “Allgemeine Depressionsskala” 14 to systematically record pain and depressive symptoms. Pain intensity was determined on a numeric rating scale (0 = no pain, 10 = maximum pain). To detect common causes of neuropathy, we performed laboratory tests including full blood count, electrolytes, kidney and liver function tests, thyroid stimulating hormone, vitamin B12, HbA1c, oral glucose tolerance test (OGTT), screening for autoimmune antibodies (i.e. ANA, ENA, ANCA), and lumbar puncture.
The study was approved by the Ethics Committees of the Universities of Würzburg (#135/15) and Erlangen (#4361, for microneurography) Medical Faculties, Germany, and study participants gave written informed consent before inclusion.
Small fiber tests
All data were processed in a blinded manner. Subjects underwent complete QST (Somedic, Hörby, Sweden) on the dorsum of the right foot following the standardized protocol of the German Research Network on Neuropathic Pain (Deutscher Forschungsverbund Neuropathischer Schmerz). 15 QST results were individually compared with published reference values based on data from 180 healthy controls spanning an age range of 20–70 years. 16 For a better reflection of the demographics of our patient cohort, we additionally compared data of the entire patient group with our own control cohort consisting of 302 healthy subjects (174 women, 128 men, median age 50 years, 17–89 years), all examined in the same QST laboratory at our department after exclusion of polyneuropathy. For the purpose of small fiber function, QST was considered abnormal if thermal detection thresholds or the thermal sensory limen were elevated. 5
Six-millimeter skin punch biopsies were obtained from the right lateral lower leg and upper thigh. One half of the biopsy specimen was used to determine IENFD following standardized procedures,17,18 while the second half was preserved for cell culture and molecular analysis. 19 IENFD results were compared with the normative values from our laboratory based on 180 healthy controls (124 women; median age: 50 years, 20–84; 56 men; median age 53 years, 22–76) in skin biopsies obtained from the lower leg (women: n = 109; men: n = 46) and the upper thigh (women: n = 102; men: n = 31). All biopsy samples were processed and assessed in our laboratory.
Study participants underwent corneal confocal microscopy (CCM) after slit lamp examination by an ophthalmologist to exclude corneal pathology. 20 The Heidelberg Retina Tomograph Rostock Cornea Module (Heidelberg Engineering, Heidelberg, Germany) was used to obtain central images of the sub-basal nerve plexus and three images per eye were selected for analysis in a blinded manner. The control group consisted of 54 women and 13 men (women: median age 50 years, range 22–65; men: median age 48 years, range 21–65).
Electrically evoked pain-related evoked potentials (PREPs) were recorded as described previously 21 applying concentric planar electrodes (Inomed Medizintechnik GmbH, Lübeck, Germany) for A-delta fiber stimulation. 22 Data were compared with our laboratory’s normal values from 90 female (median age 53 years, 22–82 years) and 59 male healthy controls (median age 47 years, 20–78 years).
Sympathetic sudomotor function was assessed on the lateral dorsum of the foot using Q-Sweat (Quantitative Sweat Measurement System, WR Medical Electronics Co, Maplewood, MA, USA) for quantitative sudomotor axon reflex test (QSART) according to the manufacturer’s protocol. Data were compared with values obtained from 17 healthy female (median age 48 years, 22–64 years) and 10 male controls (median age 56 years, 21–65 years). Means and standard deviations of the control cohorts and cut-off values are given in the legend of Table 1.
Table 1.
Characterization of the patient cohort.
| N = 86 | |
| Gender, F/M | 51/35 |
| Age, years | 53 (19–78) |
| BMI, kg/m2 | 26 (17–42) |
| Time since diagnosis, years | 0.5 (<1 month–12) |
| Pain duration, years | 4 (<1 month–25) |
| Pain distribution | |
| Acral | 27 (31%) |
| Generalized | 42 (49%) |
| Other, e.g. proximal, back | 17 (20%) |
| Pain dynamics | |
| Permanent pain with intermittent increases | 42 (49%) |
| Permanent | 26 (30%) |
| Attacks | 18 (21%) |
| Main pain descriptors | |
| Stabbing | 72 (84%) |
| Burning | 63 (73%) |
| Paresthesias in painful area | 68 (79%) |
| NPSI | |
| Burning score | 5 (0–9) |
| Pressure score | 3 (0–10) |
| Number of attacks | 2 (0–5) |
| Evoked pain score | 2 (0–9) |
| Par-/dysesthesia score | 5 (0–10) |
| Sum score | 3 (0–9) |
| GCPS | |
| Current pain intensity, NRS | 4 (0–10) |
| Max. pain intensity, NRS | 8 (0–10) |
| Mean pain intensity, NRS | 5 (0–10) |
| Days without regular activity | 14 (0–180) |
| Impairment everyday life, NRS | 3 (0–10) |
| Impairment leisure, NRS | 5 (0–10) |
| Impairment work, NRS | 5 (0–10) |
| Pain intensity sum score | 57 (0–100) |
| Disability due to pain score | 43 (0–100) |
| PCS sum score | 22 (0–52) |
| ADS sum score | 16 (0–38) |
| Analgesic medication | |
| None | 27 (31%) |
| Monotherapy | 31 (36%) |
| Combination of ⩾2 | 17 (20%) |
| Employment status | |
| Regularly working | 44 (51%) |
| Retired due to pain | 3 (3%) |
| Autonomic dysfunction, patient report | |
| Voiding problems | 15 (17%) |
| Sexual dysfunction | 13 (15%) |
| Repetitive syncope | None |
| Dyshidrosis | 46 (53%) |
| Trophic changes, patient report | |
| Skin | 16 (17%) |
| Hair | 5 (6%) |
| Nails | 7 (8%) |
| HbA1c, ref.: ⩽6.1% | 5.5 (3.6–7.7; 7 pathological) |
| 2 h OGTT, ref.: ⩽140 mg/dl | 121 (66–284; 20 pathological) |
| TSH, ref.: 0.3–4.0 mIU/l | 1.6 (0.1–8.7; 4 pathological) |
| Vitamin B12, ref.: ⩾197 pg/ml | 469 (137–1573; 2 pathological) |
| Positive autoimmune antibody finding | 14 (16%) |
| Sural nerve SNAP, µV | 17 (5–50) |
| Sural nerve NCV, m/s | 47 (41–58) |
| Tibial nerve dist. CMAP, mV | 19 (5–35) |
| Tibial nerve prox. CMAP, mV | 15 (6–28) |
| Tibial nerve NCV, m/s | 46 (35–67) |
| Tibial nerve dml, ms | 4 (3–6) |
| Idiopathic SFN | 48 (56%) |
| Impaired glucose metabolism | 15 (17%) |
| Genetic variant | 14 (16%) |
| SCN9: 2× | |
| SCN10A: 3× | |
| SCN11A: 3× | |
| NGF, TRPA1, TRPM3, TRPV3, FBLN5, SPTLC1: 1× each | |
| Other etiology | 9 (10%) |
| Pathological findings in neurological examination, n | 53 (60%) |
| Thermal hypoesthesia: | 23× |
| Mechanical hypoesthesia: | 23× |
| Hyperalgesia: | 16× |
| Hypoalgesia: | 9× |
| Allodynia: | 8× |
| Pinprick-hypoesthesia: | 2× |
| Paresthesia upon touch: | 2× |
| Hyperesthesia: | 1× |
| QST pathological, n | 22 (26%) |
| CDT | −4 (−22 to −0.8) |
| WDT | 8 (1–18) |
| TSL | 15 (4–40) |
| Distal IENFD pathological i , n | 60 (70%) |
| Proximal IENFD pathological ii , n | 39 (45%) |
| Distal IENFD, fibers/mm | 5 (0–16) |
| Proximal IENFD, fibers/mm | 9 (2–16) |
| n = 55 subgroup additionally undergoing CCM, PREP, QSART | |
| Gender, F/M | 34/21 |
| Age, years | 52 (19–78) |
| CCM pathological iii , n | 27/51 (53%) |
| CNFD, n/mm2 | 23 (2–37) |
| CNFL, mm/mm2 | 13 (5–20) |
| CNBD, n/mm2 | 47 (7–102) |
| PREP pathological iv , n | 24/51 (47%) |
| PREP N1, ms | 205 (103–293) |
| PREP P1, ms | 269 (130–335) |
| PREP PPA, µV | 0.01 (0.01–0.03) |
| QSART pathological v , n | 4/51 (8%) |
| QSART baseline, µl | 69 (20–270) |
| QSART total sweat volume, µl | 0.7 (0.05–2.4) |
| QSART response latency, s | 122 (6–600) |
Data are given as median and range in brackets, if not otherwise specified.
Means and standard deviations of the control cohorts and cut-off values:
Distal IENFD: women: mean IENFD: 8.2 ± 2.8 fibers/mm, men: 7.1 ± 2.3 fibers/mm, lower limit women: 5.4 fibers/mm, men: 4.8 fibers/mm.
Proximal IENFD: women: mean IENFD: 11.8 ± 3.3 fibers/mm, men: 10.9 ± 3.6 fibers/mm, lower limit women: 8.5 fibers/mm, men: 7.3 fibers/mm.
CCM: women: mean CNFD: 25.9 ± 6.7 n/mm2, CNFL: 14.7 ± 3.6 mm/mm2, CNBD: 78.6 ± 42.3 n/mm2, men: CNFD: 27.5 ± 4 n/mm2, CNFL: 15 ± 2.6 mm/mm2, CNBD: 67.1 ± 26.5 n/mm2. Limits: women: CNFD lower limit 19.3 n/mm2, CNFL lower limit 11.1 mm/mm2, CNBD lower limit 36.3 n/mm2, men: CNFD lower limit 23.5 n/mm2, CNFL lower limit 12.5 mm/mm2, CNBD lower limit 40.6 n/mm2.
PREP (stimulation at foot): women: mean N1: 189.92 ± 34.97 ms, P1: 245.70 ± 39.76 ms, PPA: 0.03 ± 0.02 µV, men N1: 196.13 ± 36.58 ms, P1: 248.98 ± 41.12 ms, PPA: 0.02 ± 0.01 µV, women: N1 upper limit 224.89 ms, P1 upper limit 285.46 ms, PPA lower limit 0.01 µA; men: N1 upper limit 232.71 ms, P1 upper limit 290.1 ms, PPA lower limit 0.01 µV.
QSART (measurement at feet): women: mean baseline rate: 79.86 ± 58.34 nl/min, total sweat volume 0.46 ± 0.4 µl, response latency 130.1 ± 68.9 s, men: mean baseline rate: 61.72 ± 58.34 nl/ml, total sweat volume: 0.38 ± 0.29 µl, response latency 161 ± 71.7 s, women: baseline rate 21.61 nl/ml, total volume lower limit 0.10 µl, response latency upper limit 199.10 s, men: baseline rate lower limit 47.41 nl/ml, total volume lower limit 0.85 µl, response latency upper limit 232.7 s.
ADS, Allgemeine Depressionsskala (German version of the Center for Epidemiological Studies Depression scale questionnaire); BMI, body mass index; CCM, corneal confocal microscopy; CDT, cold detection threshold; CMAP, compound motor action potential; CNBD, corneal nerve branch density; CNFD, corneal nerve fiber density; CNFL, corneal nerve fiber length; dist., distal; dml, distal motor latency; F, female; GCPS, Graded Chronic Pain Scale; HbA1c, hemoglobin A1c; IENFD, intraepidermal nerve fiber density; M, male; N1, N1 latency; NCV, nerve conduction velocity; NPSI, Neuropathic Pain Symptom Inventory; NRS, numeric rating scale; OGTT, oral glucose tolerance test; P1, P1 latency; PCS, Pain Catastrophizing Scale; PPA, peak-to-peak amplitude; PREP, pain related evoked potentials; prox., proximal; QSART, quantitative sudomotor axon reflex test; QST, quantitative sensory testing; ref., reference; SFN, small fiber neuropathy; SNAP, sensory nerve action potential; TSH, thyroid stimulating hormone; TSL, thermal sensory limen; WDT, warm detection threshold.
Microneurography was recorded from C-fibers of the superficial peroneal nerve and data were analyzed as previously described and compared with control values obtained from healthy volunteers investigated by the same examiner (B.N.).23,24
Finally, next-generation gene-panel sequencing was performed (Agilent Haloplex Panel and Illumina Nextera Rapid Capture Kit, respectively) covering the following pain-associated loci: AAAS, ARL6IP1, ATL1, ATL3, CLTCL1, DNMT1, DST, FAM134B, FLVCR1, GLA, GMPPA, IKBKAP, KIF1A, NAGLU, NGF, NTRK1, PRDM12, RAB7A, SCN9A, SCN10A, SCN11A, SPTLC1, SPTLC2, TRPA1, TTR, WNK1. For detailed methodological description, please see Supplemental material Methods online.
Statistical analysis
We used SPSS 26 (IBM, Ehningen, Germany). For non-normally distributed data we applied the non-parametric Mann–Whitney U test and expressed values as median and range. A Student’s t-test was calculated to compare the normally distributed z-scores of QST data giving mean values and standard deviation. Categorical data between groups were assessed with the χ 2 test. Microneurography data were assessed using STATISTICA 7.0 software (StatSoft Inc., Tulsa, OK, USA). Statistical significance was assumed at p < 0.05.
Results
Clinical, laboratory, and electrophysiological characteristics
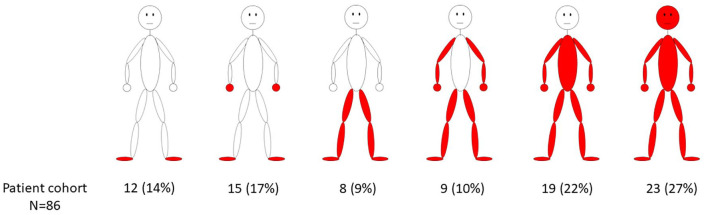
A comprehensive characterization of the study cohort including detailed pain characteristics and autonomic function complaints is presented in Table 1. The Supplemental table summarizes individual pain medication. As for pain phenotype, 27/86 (31%) patients had typical acral pain, while the rest of the patients reported widespread to generalized pain including the face and head (Figure 1). In 48/86 (56%) patients, laboratory testing was unremarkable. In 15/86 (17%) patients, the HbA1c (>6.1%) and/or OGTT (2 h glucose >140 mg/dl) were pathological, hence, impairment of glucose metabolism was newly diagnosed. In 9/86 (10%) patients, hypothyroidism, vitamin B12 deficiency, elevated autoantibody titers, and a combination of psoriasis, Hashimoto thyroiditis, and vitiligo were newly found.
Figure 1.
Pain distribution in study cohort. The drawings illustrate the frequency of pain reported in different body areas in the study cohort.
Current diagnostic criteria did not allow SFN diagnosis in almost half the study population
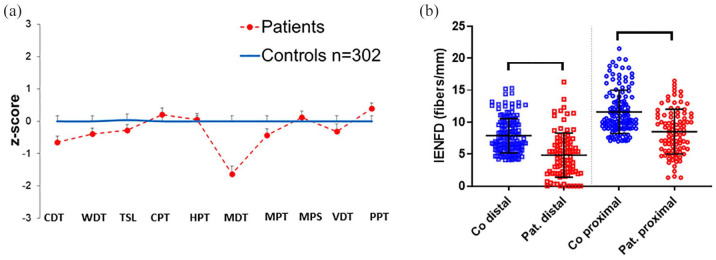
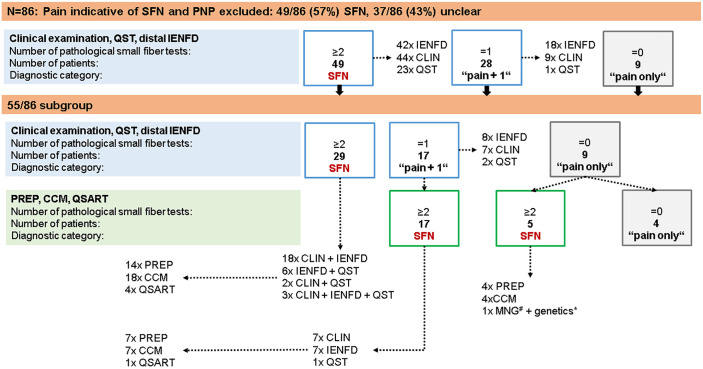
In our cohort of 86 patients, we first based the diagnosis of SFN on ⩾2 pathological small fiber tests including neurological examination, thermal thresholds on QST, and distal IENFD as recommended by Devigili et al. in a previous 4 and a recent 5 study. Distal IENFD was reduced in 60/86 (70%) patients and neurological examination was abnormal in 53/86 (62%) patients, including thermal, mechanical, and pin-prick hypoesthesia, hypo- and hyperalgesia, and touch dysesthesia (Table 1). In 38/86 (44%) patients, both tests were pathological. Proximal IENFD was reduced in only 4/86 (5%) patients with a normal distal IENFD. QST was abnormal 16 (i.e. ⩾1 cold detection threshold, warm detection threshold, thermal sensory limen) in 24/86 (28%) patients. QST data and IENFD comparing patients with the healthy control group from our laboratory are presented in Figure 2. Interestingly, QST revealed elevated mechanical detection thresholds in patients compared with controls (31/86, 36%) when comparing data with published normative values, 16 and 30/86, 35%, when comparing data with our laboratory control cohort; p < 0.01. There was no evidence of large fiber involvement in the medical history, neurological examination, and nerve conduction studies. Using currently proposed criteria for the diagnosis of SFN,4,5 49/86 (57%) patients had pathological findings in ⩾2 tests. Thus, 37/86 (43%) patients remained undiagnosed with 28/86 (33%) patients showing pathological findings in only one test (“pain+1”) and 9/86 (10%) patients without abnormal test results (“pain only”) (Figure 3).
Figure 2.
QST and IENFD results in patients with suspected SFN compared with healthy controls. (a) The z-score shows elevated MDT in patients with suspected SFN compared with healthy controls (p < 0.01). Data of 86 patients are compared with those of 302 healthy controls all investigated at the dorsal right foot. (b) The scatter plots illustrate reduced distal and proximal IENFD (p < 0.001) in patients compared with healthy controls.
CDT, cold detection threshold; Co, controls; CPT, cold pain threshold; HPT, heat pain threshold; IENFD, intraepidermal nerve fiber density; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; Pat., patients; PPT, pressure pain threshold; QST, quantitative sensory testing; SFN, small fiber neuropathy, TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold.
Figure 3.
Synopsis of study cohort and results of small fiber test results. Eighty-six patients were included and, upon exclusion of PNP, underwent neurological examination, skin punch biopsy, and QST. Depending on the number of pathological test results, patients were stratified in the subgroups of “SFN”, “pain+1” (i.e. only one small fiber test was pathological), and “pain only” (i.e. small fiber tests were normal). Fifty-five patients agreed to additionally undergo CCM, PREP, and QSART. #One patient agreed to MNG. *Genetic testing including 26 pain-associated genes (see Methods) was performed in all 86 study participants.
CCM, corneal confocal microscopy; CLIN, clinical examination; IENFD, intraepidermal nerve fiber density; MNG, microneurography; PNP, polyneuropathy; PREP, pain-related evoked potentials; QSART, quantitative sudomotor axon reflex test; QST, quantitative sensory testing; SFN, small fiber neuropathy.
Four skin innervation patterns in SFN and healthy controls
We found four innervation patterns in the skin punch biopsies of our patients and healthy controls: normal innervation, distally reduced IENFD, proximally reduced IENFD, and distally and proximally reduced IENFD (Figure 4). Of note, 37/86 (43%) patients had normal distal IENFD and 29/180 (16%) healthy controls had a reduced distal IENFD. Hence, normal innervation may not exclude SFN and reduced IENFD at the lower leg does not prove SFN.
Figure 4.
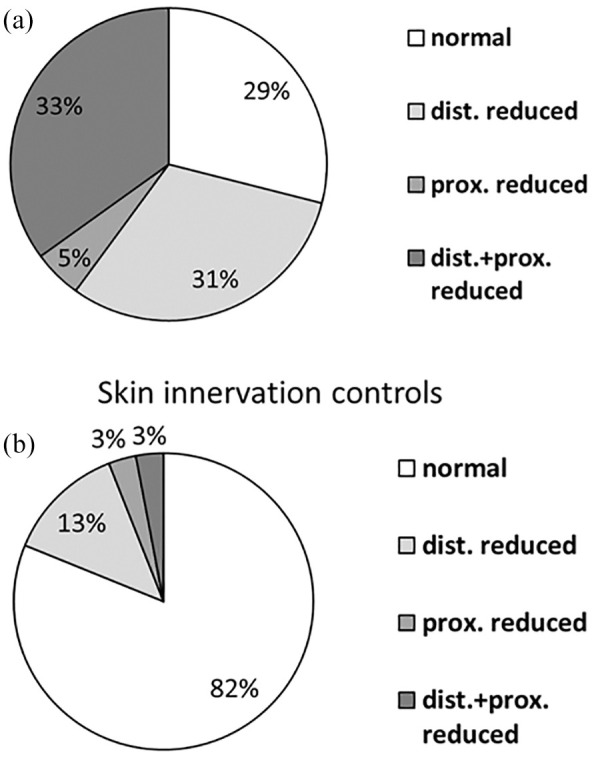
Skin innervation patterns in healthy controls and patients with suspected SFN. (a) Skin innervation was reduced at the distal leg, proximal thigh, or both sites in 71% of patients with suspected SFN. (b) Skin innervation was reduced at the distal leg, proximal thigh, or both sites in 18% of healthy controls.
Dist., distal; Prox., proximal; SFN, small fiber neuropathy.
CCM and PREP improve the detection of small fiber pathology
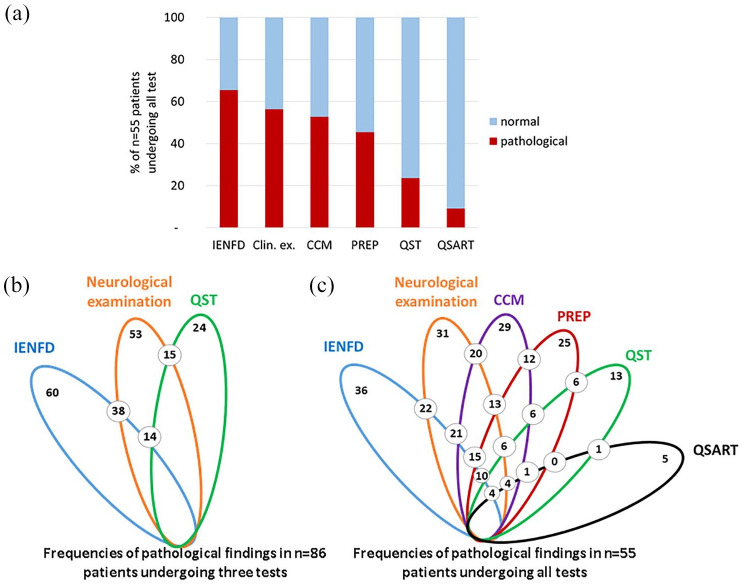
Fifty-five patients in our cohort also agreed to additionally undergo CCM, PREP, and QSART (n = 34 women, n = 21 men, median age 52 years, range 19–78; Supplemental Figure). Applying clinical examination, distal IENFD, and QST, 29/55 (53%) of these patients were already diagnosed with SFN, 17/55 (31%) patients had “pain+1”, and 9/55 (16%) had “pain only” (Figure 3). CCM showed pathological results (i.e. ⩾1 of nerve fiber length, nerve fiber density, nerve branch density) in 29/55 (53%) patients. PREP was pathological (i.e. ⩾1 of N1 latency, P1 latency, peak-to-peak amplitude) in 25/55 (45%) patients. QSART was pathological (i.e. ⩾1 of baseline rate, sweat volume, response latency) in only 5/55 (9%) patients (Figure 3). Adding these test results to those of neurological examination, QST, and distal IENFD, and using the rule of ⩾2 pathological small fiber tests, SFN was diagnosed in 49/55 (89%) patients, while 2/55 (4%) patients were categorized as “pain+1” and 4/55 (7%) patients as having “pain only”. Hence, additionally applying CCM and PREP left only 6/55 (11%) patients undiagnosed. Proximal IENFD, QST, and QSART were of limited additional value for making the diagnosis of SFN. Of note, proximal IENFD is valuable to distinguish between length-dependent (31% of our cohort) and non-length-dependent SFN (33% of our cohort). Figure 5 gives details on frequencies and distribution of pathological test results applying only three small fiber tests in the entire cohort and of six small fiber tests in this sub-cohort of 55 patients.
Figure 5.
Contribution of individual and combined tests of small fiber pathology in patients with suspected SFN. (a) The bar graphs show the frequency of pathological results in individual small fiber tests of 55 patients with suspected SFN. The lotus-shaped figures illustrate the results of the entire study cohort of 86 patients, who underwent clinical examination, skin punch biopsy, and QST (b) and the 55 patients who additionally underwent CCM, PREP, and QSART (c). Colors code for each of these investigations represented by ellipses. The number of pathological results in two small fiber tests (i.e. pairwise comparison) are given with the numbers located at the respective crossing points of the ellipses.
CCM, corneal confocal microscopy; Clin. ex., clinical examination; IENFD, intraepidermal nerve fiber density; PREP, pain-related evoked potentials; QSART, quantitative sudomotor axon reflex test; QST, quantitative sensory testing; SFN, small fiber neuropathy.
SFN may be present in spite of normal standard small fiber test results
Of the 9/55 patients in the “pain only” group, one woman agreed to microneurography (#1004). Among the three C-fibers recorded, two fibers were classified as normal mechanosensitive C-fibers, while one mechanosensitive fiber was spontaneously active with unusual huge bursting responses indicating small fiber pathology. Interestingly, this patient had a SCN10A gene variant, hence, microneurography was the only pathological small fiber test in this patient.
Genetic alterations in pain-associated genes are frequent, but mostly remain of unknown pathogenicity
Applying a panel of 26 pain-associated genes, we found 14/86 (16%) index patients who carried a genetic variant (Table 2). In 8/14 (57%) of these cases, the variation was located in a gene coding for voltage-gated sodium channels. In all cases, pathogenicity of the genetic alteration remained unclear.
Table 2.
Characterization of patients with genetic findings.
| ID | Sex | Age | Gene | Genetic variant | Pain distribution | Main pain character | Max. pain intensity (NRS) |
|---|---|---|---|---|---|---|---|
| 1 | M | 32 | SCN9 | c.4942G>A, p.(Ala1648Thr) | Feet | Burning | 8 |
| 2 | M | 31 | SCN9 | c.3911T>C, p.(Ile1304Thr) | Generalized | Burning | 6 |
| 3 | F | 58 | SCN10A | c.1094C>A, p.Thr265Asn | Generalized | Burning | 10 |
| 4 | M | 45 | SCN10A | c.3417G>C, p.(Trp1139Cys) | Generalized | Burning | 7 |
| 5 | F | 38 | SCN10A | c.5216_5217delinsTT, p.(Asp1739Val) | Generalized | Burning | 7 |
| 6 | M | 52 | SCN11A | c.603T>G, p.(Ile201Met) | Generalized | Burning | 4 |
| 7 | M | 70 | SCN11A | c.5063C>T, p.(Ala1688Val) | Generalized | Burning | 10 |
| 8 | F | 50 | SCN11A | c.1043C>A, p.(Ser348Tyr) | Feet | Burning | 8 |
| 9 | F | 46 | NGF | c.608C>T, p.(Thr23Met) | Generalized | Burning | 10 |
| 10 | M | 45 | TRPA1 | c.1678C>G, p.(His560Asp) | Generalized | Burning | 10 |
| 11 | M | 51 | TRPM3 | c.T4337T>C, p.(Ile1446Thr) | Generalized | Burning | 9 |
| 12 | F | 53 | TRPV3 | c.A958G, p.(Met320Val) | Feet+legs | Burning | 5 |
| 13 | M | 49 | FBLN5 | c.212G>A, p.(Arg71Gln) | Generalized | Burning | 8 |
| 14 | F | 50 | SPTLC1 | c.250A>G, p.(Ile84Val) | Generalized | Burning | 9 |
F, female; FBLN5, fibulin-5; M, male; NGF, nerve growth factor; NRS, numeric rating scale; SCN, voltage gated sodium channel; SPTLC1, serine palmitoyltransferase long chain base subunit 1; TRPA1/TRPM3/TRPV3, transient receptor potential ion channel sub-families.
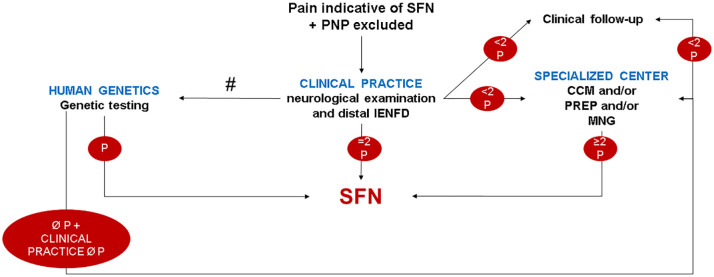
Algorithm for clinical practice
Our data show that the neurological examination is a valuable diagnostic indicator of small fiber pathology in a significant proportion (53/86, 62%) of patients with suspected SFN. Of the objective quantitative small fiber tests, distal IENFD was most informative (60/86, 70%) followed by CCM (28/55, 51%) and PREP (25/55, 45%; Figure 4). We propose an algorithm for clinical practice that may help stratify patients according to the results at each examination step (Figure 6). We keep the requisite of ⩾2 pathological small fiber test results, as a single test may also be abnormal in healthy controls.
Figure 6.
Suggested algorithm for clinical practice. The flow chart shows an algorithm which may be used in clinical practice when investigating patients with suspected SFN and which includes the most informative small fiber tests in our study. #Genetic testing possible at each diagnostic level, if not performed directly upon clinical examination.
CCM, corneal confocal microscopy; IENFD, intraepidermal nerve fiber density; MNG, microneurography; P, pathological; PNP, polyneuropathy; PREP, pain-related evoked potentials; SFN, small fiber neuropathy.
Discussion
SFN is a frequently suspected diagnosis in patients with pain of uncertain origin, in particular if pain is acrally accentuated. Currently, QST and distal IENFD are advocated and widely applied for diagnosing SFN,4,5 although some rely solely on reduced distal IENFD as the “gold standard” for SFN. 25 Here we have undertaken a detailed phenotyping study in a cohort of patients with suspected SFN, to assess the utility of six small fiber tests and genetic testing for detecting small fiber pathology or dysfunction. Among these tests, whilst distal skin punch biopsy and clinical examination were the most frequently abnormal, QST was normal in the majority of cases as reported in previous studies.26,27 This questions the diagnostic value of QST, especially as small fiber pathology was detectable with a neurological examination and underlines its importance in the diagnosis of SFN.2,4 Our data are supported by a recent study showing that a combined abnormality in clinical signs and abnormal QST and/or distal IENFD can more reliably lead to the diagnosis of SFN than the combination of abnormal QST and IENFD in the absence of clinical signs. 5 This is encouraging, since a neurological examination can be performed by every physician seeing patients with SFN. Similar to our previous studies,17,21,26,28 QST revealed elevated mechanical thresholds in our patient group, despite lack of evidence for large nerve fiber impairment in the medical history, neurological examination, and nerve conduction studies. We hypothesize that impairment of C-tactile afferents, a subgroup of unmyelinated C-nerve fiber, 29 may contribute to this finding. 30
The additional diagnostic value of CCM and PREP is appealing, given the non-invasive nature and increased yield of patients diagnosed with SFN after applying these tests. CCM 31 and PREP32,33 are well established methods; both tests showed comparable performance in the detection of small fiber pathology and were more informative than QST. In contrast, QSART was normal in almost all patients and made a minimal contribution to SFN diagnosis. Indeed, data on the utility of QSART in the assessment of small nerve fibers are contradictory.34–37 Of relevance, the majority of our patients did not have autonomic symptoms. Similarly, a proximal skin punch biopsy did not increase the diagnostic yield in our patients, since the exclusive reduction in proximal IENFD was rare. However, assessment of proximal IENFD remains of value to determine length-dependence of skin denervation. 8 Based on these data and clinical practicability, we suggest that a neurological examination and distal skin punch biopsy are required when SFN is suspected and a large fiber neuropathy has been excluded. If the diagnosis cannot be made, referral to a specialist center that provides CCM and/or PREP and/or microneurography may be considered, or the patient may be followed up. The decision of when to take which next step during the diagnostic procedure depends on the medical and individual circumstances (in terms of, e.g., symptom dynamics and severity, patient’s individual expectations), and availability of respective centers.
Similar to previous reports, 38 the etiology of SFN was idiopathic in the majority of our patients, but a substantial proportion had impaired glucose metabolism. As described in previous studies, 38 we found autoantibodies in serum samples of several patients; however, their pathophysiological role remains to be elucidated. Genetic testing should be performed early and irrespective of the family history. We detected potentially pathogenic genetic variants in 14/86 (16%) patients, which is similar to published data.38,39 Our study further underscores the importance of not restricting pain genetic studies to sodium channels and to also consider genetic testing in patients with other potential reasons for their symptoms, such as impaired glucose metabolism. Our genetic test covered 26 core genes, which have been linked to painful neuropathies. Expanding the analysis by whole-exome or whole-genome sequencing may increase the number of genetically determined small fiber neuropathies.
Patients may have typical symptoms of SFN, but normal clinical and small fiber evaluation as recently reported in a patient with a pathogenic SCN11A mutation. 40 Interestingly, our index patient with a different mutation in the SCN10A gene (#1004) also showed no small fiber pathology (“pain only”), but had an abnormality on microneurography, which is currently the most precise method for in vivo assessment of C fiber nociceptors. In contrast, SFN may be present in patients without typical symptoms or clinical signs as reported in patients with diabetes mellitus. 41 Hence, our data add to the growing evidence that SFN may be associated with varying clinical, morphological, and electrophysiological manifestations, and a diagnostic “SFN gold standard” may not be appropriate in individual cases.
Although we have applied an extensive suite of six small fiber tests, it is important to note that these investigations were performed on very small (a few millimeters to centimeters) body areas and hence cannot reflect the entire nerve fiber population. Furthermore, a value which is abnormal compared to a healthy control group does not necessarily equate to pathology. Although several small fiber tests were assessed, it is unknown which one parameter or combination of parameters underlie SFN and what is their relative contribution to the final diagnosis. Despite the application of several established pain questionnaires and a detailed documentation of patients’ pain history, a “typical” SFN pain history did not emerge. Indeed, increasing the number of pain histories emphasized the diversity of pain presentations in SFN, ranging from focal to generalized pain. The relatively large proportion of patients with a generalized pain pattern may be due to our setting as a tertiary center specialized in SFN, while more typical SFN patients may not be referred to us. Patients were recruited at one single center; however, this guaranteed standardized assessment of all study participants excluding confounding site effects. Autonomic evaluation was restricted to QSART and subjective patient reported symptoms. Another limitation is that we did not have one single healthy control group, but a separate control group for each test method. We only used PREP to investigate A-delta fiber conduction and could not address whether other ways of generating small fiber evoked potentials, for example by laser or contact-heat, may be more informative.
Our extensive phenotyping and genotyping study in a large group of patients recruited at one medical center provides robust evidence for the utility of established and novel small fiber tests in the diagnosis of SFN. We introduce an algorithm for clinical practice including the place for new small fiber tests and emphasize the need to consider SFN even if standard tests are normal.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_17562864211004318 for Diagnosing small fiber neuropathy in clinical practice: a deep phenotyping study by Nadine Egenolf, Caren Meyer zu Altenschildesche, Luisa Kreß, Katja Eggermann, Barbara Namer, Franziska Gross, Alexander Klitsch, Tobias Malzacher, Daniel Kampik, Rayaz A. Malik, Ingo Kurth, Claudia Sommer and Nurcan Üçeyler in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-pdf-2-tan-10.1177_17562864211004318 for Diagnosing small fiber neuropathy in clinical practice: a deep phenotyping study by Nadine Egenolf, Caren Meyer zu Altenschildesche, Luisa Kreß, Katja Eggermann, Barbara Namer, Franziska Gross, Alexander Klitsch, Tobias Malzacher, Daniel Kampik, Rayaz A. Malik, Ingo Kurth, Claudia Sommer and Nurcan Üçeyler in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-3-tan-10.1177_17562864211004318 for Diagnosing small fiber neuropathy in clinical practice: a deep phenotyping study by Nadine Egenolf, Caren Meyer zu Altenschildesche, Luisa Kreß, Katja Eggermann, Barbara Namer, Franziska Gross, Alexander Klitsch, Tobias Malzacher, Daniel Kampik, Rayaz A. Malik, Ingo Kurth, Claudia Sommer and Nurcan Üçeyler in Therapeutic Advances in Neurological Disorders
Acknowledgments
We thank Barbara Broll, Philine Dinkel, Danilo Prtvar, and Daniela Urlaub for expert technical assistance, and Dr. Julian Roth for inspiring Figure 5.
Footnotes
Conflict of interest statement: N.S., C.M.z.A., L.K., F.G., K.E., B.N., T.M., A.K., D.K., I.K. report no conflicts of interest. C.S., R.A.M., N.Ü. have taken part in clinical studies associated with small fiber neuropathy (Biogen, Vertex).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; N.Ü.: UE171-3/1). B.N. (NA970 3-1) and N.Ü. (UE171-5/1) were supported by the DFG. This publication was supported by the Open Access Publication Fund of the University of Würzburg.
ORCID iD: Nurcan Üçeyler  https://orcid.org/0000-0001-6973-6428
https://orcid.org/0000-0001-6973-6428
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Nadine Egenolf, Department of Neurology, University of Würzburg, Germany.
Caren Meyer zu Altenschildesche, Department of Neurology, University of Würzburg, Germany.
Luisa Kreß, Department of Neurology, University of Würzburg, Germany.
Katja Eggermann, Institute of Human Genetics, Medical Faculty, RWTH Aachen University, Aachen, Nordrhein-Westfalen, Germany.
Barbara Namer, Institute of Physiology, University of Erlangen, Bayern, Germany.
Franziska Gross, Department of Neurology, University of Würzburg, Germany.
Alexander Klitsch, Department of Neurology, University of Würzburg, Germany.
Tobias Malzacher, Department of Neurology, University of Würzburg, Germany.
Daniel Kampik, Department of Ophthalmology, University of Würzburg, Bayern, Germany.
Rayaz A. Malik, Weill Cornell Medicine-Qatar, Qatar Foundation, Education City, Doha, Qatar
Ingo Kurth, Institute of Human Genetics, Medical Faculty, RWTH Aachen University, Aachen, Nordrhein-Westfalen, Germany.
Claudia Sommer, Department of Neurology, University of Würzburg, Germany.
Nurcan Üçeyler, Department of Neurology, University of Würzburg, Josef-Schneider-Str. 11, Würzburg, 97080, Germany.
References
- 1. Terkelsen AJ, Karlsson P, Lauria G, et al. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol 2017; 16: 934–944. [DOI] [PubMed] [Google Scholar]
- 2. Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002; 26: 173–188. [DOI] [PubMed] [Google Scholar]
- 3. Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve 1992; 15: 661–665. [DOI] [PubMed] [Google Scholar]
- 4. Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008; 131: 1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devigili G, Rinaldo S, Lombardi R, et al. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain 2019; 142: 3728–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haroutounian S, Todorovic MS, Leinders M, et al. Diagnostic criteria for idiopathic small fiber neuropathy: a systematic review. Muscle Nerve 2020; 63: 170–177. [DOI] [PubMed] [Google Scholar]
- 7. Haanpaa M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011; 152: 14–27. [DOI] [PubMed] [Google Scholar]
- 8. Joint Task Force of the EFNS and the PNS; Lauria G, Hsieh ST, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. J Peripher Nerv Syst 2010; 15: 79–92. [DOI] [PubMed] [Google Scholar]
- 9. Freeman R, Gewandter JS, Faber CG, et al. Idiopathic distal sensory polyneuropathy: ACTTION diagnostic criteria. Neurology 2020; 95: 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004; 108: 248–257. [DOI] [PubMed] [Google Scholar]
- 11. Sommer C, Richter H, Rogausch JP, et al. A modified score to identify and discriminate neuropathic pain: a study on the German version of the Neuropathic Pain Symptom Inventory (NPSI). BMC Neurol 2011; 11: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Von Korff M, Ormel J, Keefe FJ, et al. Grading the severity of chronic pain. Pain 1992; 50: 133–149. [DOI] [PubMed] [Google Scholar]
- 13. Meyer K, Sprott H, Mannion AF. Cross-cultural adaptation, reliability, and validity of the German version of the Pain Catastrophizing Scale. J Psychosom Res 2008; 64: 469–478. [DOI] [PubMed] [Google Scholar]
- 14. Meyer TD, Hautzinger M. Allgemeine Depressions-Skala (ADS) Normierung an Minderjährigen und Erweiterung zur Erfassung manischer Symptome (ADMS). Diagnostica 2001; 47: 208–215. [Google Scholar]
- 15. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006; 123: 231–243. [DOI] [PubMed] [Google Scholar]
- 16. Magerl W, Krumova EK, Baron R, et al. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain 2010; 151: 598–605. [DOI] [PubMed] [Google Scholar]
- 17. Üçeyler N, Kafke W, Riediger N, et al. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology 2010; 74: 1806–1813. [DOI] [PubMed] [Google Scholar]
- 18. Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010; 15: 202–207. [DOI] [PubMed] [Google Scholar]
- 19. Kreß L, Hofmann L, Klein T, et al. Differential impact of keratinocytes and fibroblasts on nociceptor degeneration and sensitization in small fiber neuropathy. Pain. Epub ahead of print 12 November 2020. DOI: 10.1097/j.pain.0000000000002122. [DOI] [PubMed] [Google Scholar]
- 20. Kalteniece A, Ferdousi M, Adam S, et al. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS One 2017; 12: e0183040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Üçeyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013; 136: 1857–1867. [DOI] [PubMed] [Google Scholar]
- 22. Lefaucheur JP, Ahdab R, Ayache SS, et al. Pain-related evoked potentials: a comparative study between electrical stimulation using a concentric planar electrode and laser stimulation using a CO2 laser. Neurophysiol Clin 2012; 42: 199–206. [DOI] [PubMed] [Google Scholar]
- 23. Hagbarth KE. Microelectrode recordings from human peripheral nerves (microneurography). Muscle Nerve Suppl 2002; 11: S28–35. [DOI] [PubMed] [Google Scholar]
- 24. Schmelz M, Forster C, Schmidt R, et al. Delayed responses to electrical stimuli reflect C-fiber responsiveness in human microneurography. Exp Brain Res 1995; 104: 331–336. [DOI] [PubMed] [Google Scholar]
- 25. Piscosquito G, Provitera V, Mozzillo S, et al. The analysis of epidermal nerve fibre spatial distribution improves the diagnostic yield of skin biopsy. Neuropathol Appl Neurobiol. Epub ahead of print 19 August 2020. DOI: 10.1111/nan.12651. [DOI] [PubMed] [Google Scholar]
- 26. Üçeyler N, Vollert J, Broll B, et al. Sensory profiles and skin innervation of patients with painful and painless neuropathies. Pain 2018; 159: 1867–1876. [DOI] [PubMed] [Google Scholar]
- 27. Scherens A, Maier C, Haussleiter IS, et al. Painful or painless lower limb dysesthesias are highly predictive of peripheral neuropathy: comparison of different diagnostic modalities. Eur J Pain 2009; 13: 711–718. [DOI] [PubMed] [Google Scholar]
- 28. Evdokimov D, Frank J, Klitsch A, et al. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol 2019; 86: 504–516. [DOI] [PubMed] [Google Scholar]
- 29. Olausson H, Lamarre Y, Backlund H, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci 2002; 5: 900–904. [DOI] [PubMed] [Google Scholar]
- 30. Morrison I, Loken LS, Minde J, et al. Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain 2011; 134: 1116–1126. [DOI] [PubMed] [Google Scholar]
- 31. Tavakoli M, Marshall A, Pitceathly R, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol 2010; 223: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Özgül OS, Maier C, Enax-Krumova EK, et al. High test-retest-reliability of pain-related evoked potentials (PREP) in healthy subjects. Neurosci Lett 2017; 647: 110–116. [DOI] [PubMed] [Google Scholar]
- 33. Üçeyler N, Kahn AK, Kramer D, et al. Impaired small fiber conduction in patients with Fabry disease: a neurophysiological case-control study. BMC Neurol 2013; 13: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thaisetthawatkul P, Fernandes JAM, Herrmann DN. Contribution of Qsart to the diagnosis of small fiber neuropathy. Muscle Nerve 2013; 48: 883–888. [DOI] [PubMed] [Google Scholar]
- 35. Kamel JT, Vogrin SJ, Knight-Sadler RJ, et al. Combining cutaneous silent periods with quantitative sudomotor axon reflex testing in the assessment of diabetic small fiber neuropathy. Clin Neurophys 2015; 126: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 36. Berger MJ, Kimpinski K. Test-retest reliability of quantitative sudomotor axon reflex testing. J Clin Neurophysiol 2013; 30: 308–312. [DOI] [PubMed] [Google Scholar]
- 37. Tavee JO, Polston D, Zhou L, et al. Sural sensory nerve action potential, epidermal nerve fiber density, and quantitative sudomotor axon reflex in the healthy elderly. Muscle Nerve 2014; 49: 564–569. [DOI] [PubMed] [Google Scholar]
- 38. de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, et al. Associated conditions in small fiber neuropathy - a large cohort study and review of the literature. Eur J Neurol 2018; 25: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brouwer BA, Merkies ISJ, Gerrits MM, et al. Painful neuropathies: the emerging role of sodium channelopathies. J Peripher Nerv Syst 2014; 19: 53–65. [DOI] [PubMed] [Google Scholar]
- 40. Castoro R, Simmons M, Ravi V, et al. SCN11A Arg225Cys mutation causes nociceptive pain without detectable peripheral nerve pathology. Neurol Genet 2018; 4: e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rage M, Van Acker N, Knaapen MWM, et al. Asymptomatic small fiber neuropathy in diabetes mellitus: investigations with intraepidermal nerve fiber density, quantitative sensory testing and laser-evoked potentials. J Neurol 2011; 258: 1852–1864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_17562864211004318 for Diagnosing small fiber neuropathy in clinical practice: a deep phenotyping study by Nadine Egenolf, Caren Meyer zu Altenschildesche, Luisa Kreß, Katja Eggermann, Barbara Namer, Franziska Gross, Alexander Klitsch, Tobias Malzacher, Daniel Kampik, Rayaz A. Malik, Ingo Kurth, Claudia Sommer and Nurcan Üçeyler in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-pdf-2-tan-10.1177_17562864211004318 for Diagnosing small fiber neuropathy in clinical practice: a deep phenotyping study by Nadine Egenolf, Caren Meyer zu Altenschildesche, Luisa Kreß, Katja Eggermann, Barbara Namer, Franziska Gross, Alexander Klitsch, Tobias Malzacher, Daniel Kampik, Rayaz A. Malik, Ingo Kurth, Claudia Sommer and Nurcan Üçeyler in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-3-tan-10.1177_17562864211004318 for Diagnosing small fiber neuropathy in clinical practice: a deep phenotyping study by Nadine Egenolf, Caren Meyer zu Altenschildesche, Luisa Kreß, Katja Eggermann, Barbara Namer, Franziska Gross, Alexander Klitsch, Tobias Malzacher, Daniel Kampik, Rayaz A. Malik, Ingo Kurth, Claudia Sommer and Nurcan Üçeyler in Therapeutic Advances in Neurological Disorders