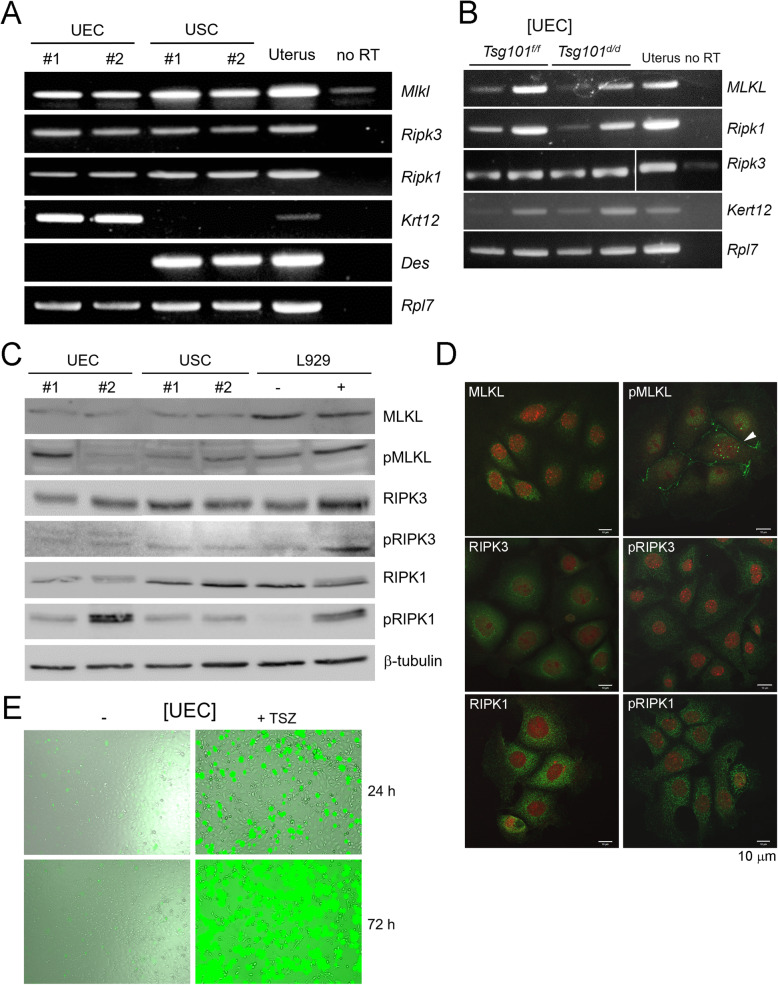
Fig. 4.
UECs express necroptosis effectors, RIPK1, RIPK3, and MLKL, and respond to necroptosis-inducing signal. (A) RT-PCR of necroptosis factors performed in isolated UECs, USCs, and uteri from random cycling ICR mice. Five mice were pooled for each group. E2 was given to mice 24 h before sacrifice to increase yield of UECs. Results from two sets of independent samples experiments are shown as #1 and #2. RNA from whole uteri was used as a positive control. Mlkl, Mixed lineage kinase domain-like; Ripk3, Receptor interacting protein kinase 3; Ripk1, Receptor interacting protein kinase 1; Des, Desmin (a stromal marker); Krt12, Keratin 12 (an epithelial marker); Rpl7, Ribosomal protein L7 (a housekeeping gene). Experiments were repeated three times with similar results. (B) RT-PCR of necroptosis effectors, Mlkl, Ripk1, and Ripk3 in UECs isolated from Tsg101f/f and Tsg101d/d mice. Two independent samples were used. Mlkl, Mixed lineage kinase domain-like; Ripk3, Receptor interacting protein kinase 3; Ripk1, Receptor interacting protein kinase 1; Des, Desmin (a stromal marker); Krt12, Keratin 12 (an epithelial marker). (C) Western blot analyses of necroptosis effectors in UECs and USCs. L929 cells treated with TSZ were used as a positive control. pMLKL, phospho-MLKL; pRIPK1, phospho-RIPK1; pRIPK3, phospho-RIPK3. Two independent samples were used (#1 and #2), and experiments were repeated two times with similar results. (D) Immunofluorescence staining of necroptosis effects in cultured UECs. DNA was stained with TO-PRO™-3-Iodine (1:250). Experiments were repeated three times; a set of representative images are shown. (E) Isolated UECs were plated and treated with TSZ (TNFα + Smac mimetic LCL161 + zVAD-fmk) or DMSO (vehicle) the day after all cells had attached. TSZ was added at 24 h in culture along with SYTOX Green dye, which stains dead cells only. Live images were captured at 1 h interval using the JuLIFM FL. TNFα, 30 ng/ml; Smac mimetic LCL161, 10 μM; zVAD-fmk, 20 μM