ABSTRACT
The need for alternative treatments for multiple sclerosis (MS) has triggered copious amounts of research into microbial therapies focused on manipulating the microbiota–gut–brain axis. This comprehensive review was intended to present and systematically evaluate the current clinical and preclinical evidence for various probiotic and commensal gut microbial therapies as treatments for MS, using the Bradford Hill criteria (BHC) as a multi-parameter assessment rubric. Literature searches were performed to identify a total of 37 relevant studies (6 human, 31 animal), including 28 probiotic therapy and 9 commensal therapy studies. In addition to presenting qualitative summaries of these findings, therapeutic evidence for each bacterial formulation was assessed using the BHC to generate summative scores. These scores, which encompassed study quality, replication, and other considerations, were used to rank the most promising therapies and highlight deficiencies. Several therapeutic formulations, including VSL#3, Lactobacillus paracasei, Bifidobacterium animalis, E. coli Nissle 1917, and Prevotella histicola, emerged as the most promising. In contrast, a number of other therapies were hindered by limited evidence of replicable findings and other criteria, which need to be addressed by future studies in order to harness gut microbial therapies to ultimately provide cheaper, safer, and more durable treatments for MS.
KEYWORDS: Multiple sclerosis, probiotics, commensals, microbiome, microbiota–gut–brain axis, autoimmune disease, comprehensive literature review
Introduction
Multiple sclerosis
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system (CNS) characterized by neuroinflammation, myelin sheath degeneration, axonal loss, and blood–brain barrier (BBB) deterioration.1,2 Globally, about 2.8 million people are estimated to live with MS.3 The disease typically follows four different clinical courses: clinically isolated syndrome (CIS), relapsing-remitting MS (RRMS), primary progressive MS (PPMS), and secondary progressive MS (SPMS), with progressive forms being most severe and refractory to treatment.1 As MS progresses, most patients are challenged with chronic pain and fatigue, gradual sensorimotor impairments, bowel and bladder dysfunction, cognitive changes, and overall diminished quality of life.1,2
There are currently 15 FDA-approved disease-modifying therapies (DMTs) used to decrease the severity and frequency of MS relapses.4–6 These DMTs are generally effective at mitigating MS pathology by suppressing various aspects of the immune system, but they are also expensive,2 often accompanied by an array of side effects,5 and demonstrate decreased efficacy over time.6–9 As such, improved, alternative MS treatments are warranted.
Like most chronic diseases, susceptibility to MS is driven by both genetic and environmental components, with the latter including well-documented risk factors like Epstein–Barr virus infection, vitamin D-insufficiency, and smoking.10,11 An emerging putative pseudo-environmental risk factor for MS and other chronic diseases is an imbalance (dysbiosis) in the gut microbiome, a complex ecosystem of trillions of microorganisms inhabiting our intestinal tracts. A generalized mechanism proposed is the bidirectional communication between the CNS and gut by way of the so-called microbiota-gut-brain axis (MGBA).1,12–14 Dysbiosis within the gut can promote effector T cell phenotypes toward proinflammatory pathways that subsequently increase intestinal barrier permeability.15–20 This enables the release of microbial antigens and intestinal immune cells into circulation, further promoting systemic, low-level inflammation which may contribute to the weakened BBB tight junctions and enhanced T cell autoreactivity observed in MS.15,16,18–20 The directionality of whether MS contributes to, as opposed to results from, this dysbiosis, however, is still unclear. Nevertheless, multiple studies16,18–20 have characterized MS gut microbiomes as distinct from their healthy control-counterparts, generally possessing an elevated relative abundance of microrganisms associated with inflammation that when transplanted into mice have been shown to exacerbate an experimental animal model of MS, experimental autoimmune encephalomyelitis (EAE).19
Commensal and probiotic gut bacterial therapies
Given the putative role of gut dysbiosis in promoting MS susceptibility, an attractive therapeutic approach would be to restore balance of the microbiome, and/or to take advantage of the intimate cross-talk between the immune system and gut microorganisms to inhibit or skew autoimmune responses.21–24 Hence, gut targeted microbial therapies have been gaining traction as alternative or supplemental treatment options for a variety of conditions, including MS.
Gut bacterial commensals are generally beneficial organisms naturally comprising the gut microbiome to maintain a healthy host environment.22 Whereas probiotics are defined by the International Scientific Association for Probiotics and Prebiotics (ISAPP) as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”25 For the purposes of this review, these are defined as live bacteria that can be supplemented into the microbiome to elicit beneficial changes in the commensal microbial community structure, and/or exert direct beneficial effects on the host.22,26,27 It should be noted, however, that the exact distinction between gut bacterial commensals and bacterial probiotics remains arbitrary and far from uniform across studies, organizations, and regulatory guidelines. Nevertheless, both commensals and probiotics have important roles in digestive and immune health, including nutrient and vitamin synthesis, metabolism of host dietary products, intestinal barrier reinforcement, prevention of pathogenic microbe colonization, and anti-inflammatory immunoregulation.21,22,26,27Consequently, both have the therapeutic potential to mitigate MS pathology through modulation of the MGBA. This, in combination with accessibility and relatively low costs, makes probiotic and commensal therapies attractive alternative MS treatment candidates.
Alternative MS therapies: where do we stand?
A number of recent reviews have attempted to compile the growing body of evidence for gut microbiome-targeted therapies, though most of these are far from comprehensive. 12,26–34 To date, there have been two published reviews that evaluate the clinical utility of probiotics and explore possible underlying mechanisms, including one systematic review of two clinical and five preclinical studies,31 and one recent study with a meta-analysis of three clinical studies and systematic review of 22 preclinical studies.33 Though valuable for highlighting probiotic therapeutic efficacy, both of these reviews are exclusively focused on probiotics without consideration of commensal therapy, and neither ranked the current evidence of each specific gut bacterial formulations using a quantitative objective rubric. Addressing the latter is particularly important, given that it helps to identify stronger and weaker areas within the field, highlight discrepancies, and ultimately provide direction for future research.
Accordingly, in this comprehensive review, we attempt to (1) compile the current clinical and preclinical evidence of MS mitigation by probiotic and commensal therapies; and (2) systematically rank the evidence of each gut-bacterial formulation using the Bradford Hill criteria (see below). In doing so, we aim to identify the most promising emerging therapies, as well as to highlight existing shortcomings in the field and emphasize specific foci for future studies.
Methods
This comprehensive review was originally intended as a systematic review, and therefore registered in the International Prospective Register of Systematic Reviews (PROSPERO; ID# CRD42020206819) following the initial search, but prior to screening articles.
Search strategies
Searches were conducted by two authors (LB & TM) on August 27, 2020 and January 4, 2021 using four databases: OvidMEDLINE, CINAHL, PubMed, and Web of Science. One paper was identified separately by one author (DK) outside of the search strategy.35 Search strategies were tailored to each database using keywords, MeSH and MH headings, truncation, and an English Language filter (Supp. File 1A).
Selection criteria
Studies from all years were included in this review if they (1) were written or available in English, (2) investigated the effects of probiotic and/or commensal therapy on MS or an MS animal model severity and progression, and (3) utilized an experimental/intervention-based study design. Studies were excluded if they did not meet the inclusion criteria and/or used a non-intervention/experimental study design, including cohorts, cross-sectional studies, case–control studies, case series, and case reports.
Operational definitions
As mentioned above, the distinction between bacterial probiotics and commensals is not well defined, particularly as it applies to MS. For the purposes of this review, a bacterial therapeutic was considered “probiotic” when meeting evidence level 1–2 based on World Gastroenterology Organization guidelines from the Oxford Center for Evidence-Based Medicine, and “commensal” if falling at evidence level 3 and below where RCTs are lacking.36 Study interventions were therefore classified as “probiotic therapy” if researchers supplemented with the following putative probiotics: Lactobacillus spp., Bifidobacterium spp., Escherichia coli Nissle 1917 (E. coli Nissle 1917), Enterococcus faecium (E. faecium), or Streptococcus thermophilus (S. thermopohilis); or “commensal therapy” if researchers supplemented with any other species of commensal bacteria, including Prevotella spp., Akkermansia spp., Pediococcus acidilactici (P. acidilactici), Clostridium butyricum (C. butyricum), and Bacteroides fragilis (B. fragilis).
There were two animal studies that were exceptions to these classifications, for the following reasons. Both studies introduced putative probiotic Lactobacillus spp. via stable colonization by a single inoculation rather than continuous treatment, which is more representative of commensal therapy than a probiotic therapy.37,38Additionally, the bacterial strains used in these two studies are not strains recognized as probiotics, but are instead isolates from commensal murine gut microbiota. These two studies were hence classified as commensal therapy.
Data extraction
Following screening studies for relevance against the selection criteria (LB, TM, & DK), data were extracted from the included studies by two authors (LB & TM) (Supp. File 1). The study metrics extracted included first author, year of publication, DOI, location of study, study design, sample, intervention, duration of study, MS model (for animal studies), measurements/outcomes, statistical methods, and power (for human studies). The study measurement/outcomes extracted included clinical parameters of MS/EAE severity and progression, immune and metabolic indices, microbiome and metabolome parameters, and mechanistic or correlative findings.
Evaluating quality and evidence of included studies
Included studies were subject to quality and risk of bias (ROB) assessments using the Cochrane ROB tool39 for human studies and SYRCLE tool40 for animal studies. High quality was assigned to studies with a low ROB, including randomized controlled trials (RCTs) and animal studies that explicitly stated using randomization and blinding measures. Medium quality was assigned to studies with an uncertain ROB, including non-RCT human studies and animal studies that did not explicitly state using randomization and/or blinding measures. Low quality was assigned to studies with a high ROB, including studies with considerable confounding, in addition to not explicitly stating the use of randomization or blinding. These quality assessments were factored into the summative evaluation of each bacterial therapy; therefore, no studies were excluded from analysis on the basis of ROB.
The overall quality and strength of therapeutic evidence provided by each bacterial formulation was assessed using the Bradford Hill criteria (BHC), which includes the following: temporal relationship, strength of relationship, dose–response relationship, replication of findings, biological plausibility, cessation of exposure, specificity of association, and coherence between multiple approaches.41,42 The descriptions and numerical designations of each BHC can be found in Table 1. Sufficient evidence (Yes or No) was determined for each criterion (except for replication, see below) and assigned a score of 1 for yes, followed by summation across all criteria to yield a final “BH score” for each therapy. Replication of findings was the most heavily weighted criterion, and was scored as follows: 3 = replicated in human and animal studies, 2 = replicated by different groups, 1 = replicated by the same group, 0 = not replicated, −2 = conflicting findings (not considering lack of effect as conflicting with positive). The calculations are detailed in Supplemental File 2 and summarized in Table 5.
Table 1.
Bradford Hill criteria numerical designations and descriptions
| BHC | BHC # | Description |
|---|---|---|
| Temporal relationship | 1 | Does the exposure precede the outcome? |
| Strength of relationship | 2 | Level of evidence, ROB, study quality |
| Dose-response relationship | 3 | U-shaped, inverse U-shaped, etc. |
| Replication of findings | 4 | Across studies, across research groups, etc. |
| Biological plausibility | 5 | Do the findings make biological sense? |
| Cessation of exposure | 6 | Do the effects change after discontinuation? |
| Specificity of association | 7 | Does the exposure lead to the outcome? |
| Coherence between multiple approaches | 8 | How well do different lines of experimentation or observation support one another? |
BHC, Bradford Hill criteria; ROB, risk of bias.
Table 5.
Bradford Hill criteria evaluation of probiotic and commensal therapies
| Therapy | Study | Study Type | ROB | Strain Used | Treatment Effect | BH Score |
|---|---|---|---|---|---|---|
| VSL#3/ Vivomixx/ LBS |
Mestre et al.43 | Animal TMEV-IDD Thera | UN | L. paracasei DSM 24734, L. plantarum DSM 24730, L. acidophilus DSM 24735, L. delbrueckii ssp. bulgaricus DSM 24734, B. longum DSM 24736, B. infantis DSM 24737, B. breve DSM 24732, S. thermophilus DSM 24731 | Improved | 9 |
| Calvo-Barreiro et al.44 | Animal EAE Thera | Low | Improved | |||
| McMurran et al.45 | Animal Cuprizone Proph & Thera | Low | No change | |||
| Tankou et al.46 | Human Prospective Cohort | High | Improved (immunologically) | |||
| Tankou et al.47 | Human Prospective Cohort | High | Improved (immunologically & microbiologically) | |||
| L. paracasei | Lavasani et al.48 | Animal EAE Proph & Thera | UN | DSM 13434, PCC 101 | Improved | 7 |
| Sanchez et al.49 | Animal EAE Proph & Thera | UN | ATCC 27092 (live & heat-killed), ATCC 11582, DSM 2649, DSM 5622, ATCC 334 | Improved | ||
| B. animalis | Salehipour et al.50 | Animal EAE Thera | Low | PTCC 1631 | Improved | 7 |
| Ezendam et al.51 | Animal EAE Proph & Thera | UN | Not specified | No change | ||
| Goudarzvand et al.52 | Animal GID Thera | UN | Susbp. lactis B94 | No change | ||
| Consonni et al.53 | Animal EAE Proph & Thera | Low | Subsp. lactis BB12 | Slightly improved | ||
| Consonni et al.53 | Animal EAE Proph & Thera | Low | Subsp. lactis LMG S-28195 | Slightly improved | ||
| E. coli | Libbey et al.54 | Animal EAE Proph | UN | Nissle 1917 recombinant strain for ampicillin resistance (pGEN-MCS) | Improved | 7 |
| Secher et al.55 | Animal EAE Proph & Thera | UN | Nissle 1917 | Improved | ||
| P. histicola | Mangalam et al.56 | Animal EAE Proph & Thera | UN | Isolated from duodenum of celiac disease patients | Improved | 7 |
| Shahi et al.57 | Animal EAE Proph & Thera | UN | Isolated from duodenum of celiac disease patients | Improved | ||
| Shahi et al.58 | Animal EAE Proph & Thera | UN | Isolated from duodenum of celiac disease patients | Improved | ||
| L. plantarum | Lavasani et al.48 | Animal EAE Proph & Thera | UN | DSM 15312, DSM 15313 | Improved | 6 |
| Salehipour et al.50 | Animal EAE Thera | Low | A7 | Improved | ||
| Goudarzvand et al.52 | Animal GID Thera | UN | Not specified | No change | ||
| Maassen et al.59 | Animal EAE Proph | UN | NCIB 8826, 14917 | No change | ||
| Lacto-mix | Lavasani et al.48 | Animal EAE Proph & Thera | UN | L. plantarum DSM 15312, L. plantarum DSM 15313, L. paracasei DSM 13434 | Improved | 6 |
| L. crispatus & L. rhamnosus | Consonni et al.53 | Animal EAE Proph & Thera | Low | L. crispatus LMG P-23257 & L. rhamnosus ATCC 53103 | Improved | 6 |
| B. animalis & B. animalis | Consonni et al.53 | Animal EAE Proph & Thera | Low | B. animalis subsp. lactis BB12 & B. animalis subsp. lactis LMG S-28195 | Improved | 6 |
| L. acidophilus, L. casei, L. fermentum, & B. bifidum | Kouchaki et al.60 | Human RCT | Low | Not specified | Improved | 5 |
| Tamtaji et al.61 | Human RCT | Low | Not specified | Slightly improved (immunologically) | ||
| B. fragilis | Ochoa-Reparaz et al.62 | Animal EAE Proph |
UN | NCTC 9343 | Improved | 5 |
| L. crispatus | Consonni et al.53 | Animal EAE Proph & Thera | Low | LMG P-23257 | Improved | 4 |
| L. rhamnosus | Consonni et al.53 | Animal EAE Proph & Thera | Low | ATCC 53103 | Improved | 4 |
| Lactibiane iki | Calvo-Barreiro et al.44 | Animal EAE Thera | Low | B. lactis LA 304, L. acidophilus LA 201, L. salivarius LA 302 | Improved | 4 |
| Protexin | Rahimlou et al.63 | Human RCT |
Low | B. subtilis PXN 21, B. bifidum PXN 23, B. breve PXN 25, B. infantis PXN 27, B. longum PXN 30, L. acidophilus PXN 35, L. rhamnosus PXN 54, L. helveticus PXN 45, L. salivarius PXN 57, L. lactis ssp. lactis PXN 63, S. thermophilus PXN 66, L. casei PXN 37, L. delbrueckii ssp. bulgaricus PXN 39, L. plantarum PXN 47 | Slightly improved | 4 |
| L. plantarum, L. casei, L. reuteri, L. fermentum, B. infantis, & B. lactis | Salami et al.64 | Human RCT | Low | Not specified | Improved | 4 |
| L. plantarum & B. animalis | Salehipour et al.50 | Animal EAE Thera | Low | L. plantarum A7, B. animalis PTCC 1631 | Improved | 4 |
| C. butyricum | Chen et al.65 | Animal EAE Proph | UN | GDBIO1501 | Improved | 4 |
| Allobaculum | Miyauchi et al.38 | Animal EAE Proph | Low | Isolated from content of small intestines of specific PF mice | Slightly worsened | 4 |
| L. murinus | Maassen et al.59 | Animal EAE Proph | UN | CNRZ | Improved | 3 |
| E. faecium | Abdurasulova et al.66 | Animal EAE Thera | UN | LMG P-27496 | Improved | 3 |
| L. helveticus | Yamashita et al.67 | Animal EAE Proph & Thera | UN | SBT2171 | Improved | 2 |
| B. breve | Kobayashi et al.68 | Animal EAE Proph & Thera | UN | Yakult | Slightly improved (F) Worsened (M) | 2 |
| IRT5 | Kwon et al.69 | Animal EAE Proph & Thera | UN | L. casei, L. acidophilus, L. reuteri, B. bifidum, S. thermophilus | Improved | 2 |
| P. acidilactici | Takata et al.70 | Animal EAE Proph & Thera | UN | R037 | Improved | 2 |
| A. muciniphila | Liu et al.35 | Animal EAE Thera | UN | ATCC BAA-835 | Improved | 2 |
| L. casei | Maassen et al.59 | Animal EAE Proph | UN | 393 | Slightly improved | 1 |
| Gharehkhani Digehsara et al.71 | Animal Cuprizone Proph & Thera | UN | Not specified | Improved | ||
| Ezendam & van Loveren72 | Animal EAE Proph & Thera | High | Shirota | Slightly worsened | ||
| Baken et al.73 | Animal EAE Proph & Thera | UN | Shirota | Worsened | ||
| Kobayashi et al.68 | Animal EAE Proph & Thera | UN | Shirota | No change | ||
| Kobayashi et al.74 | Animal EAE Proph & Thera | UN | YIT 9029 | No change | ||
| L. reuteri | He et al.75 | Animal EAE Thera | UN | DSM 17938 | Improved | 1 |
| Johanson et al.76 | Animal EAE Thera | UN | ATCC 2327 | Improved | ||
| Maassen et al.59 | Animal EAE Proph | UN | ML1 | Worsened | ||
| Miyauchi et al.38 | Animal EAE Proph | Low | Isolated from content of small intestines of specific PF mice 100% 16S rRNA match to strains H4 & LMG 18238 Also uvrA-deficient L. reuteri | Slightly worsened | ||
| Montgomery et al.37 | Animal EAE Proph | Low | Isolated from PWD cecal contents | Worsened | ||
| L. delbrueckii subsp. bulgaricus | Lavasani et al.48 | Animal EAE Proph & Thera | UN | DSM 20081 | No change | 1 |
Abbreviations: ROB, risk of bias; BH, Bradford Hill; EAE, experimental autoimmune encephalomyelitis; Proph, prophylactic; Thera, therapeutic; RCT, randomized controlled trial; GID, gliotoxin-induced demyelination; TMEV-IDD, Theiler’s murine encephalomyelitis virus-induced demyelinating disease; UN, uncertain.
Results
A. Study characteristics
The study characteristics and major findings of the included studies are summarized in Table 1–3. A total of 770 de-duplicated articles were found by the initial search, 55 additional articles were found by the second search, and one article76 was found by an author (DK) outside of the search strategy. A total of 37 studies35,37,3843–76 (6 human, 31 animal) were included for analysis in this review based on the stated selection criteria (see Methods) (Figure 1; Supp. File 1B). Of these 37 studies, 28 (6 human, 22 animal) investigated the effects of probiotic therapy and 9 (0 human, 9 animal) utilized commensal therapy. Studies were conducted between 1998 and 2020 in the following countries: USA, Iran, Japan, England, Netherlands, Spain, Russia, Italy, Sweden, France, China, and Republic of Korea.
Table 2.
Human studies of probiotic therapies: summary of study characteristics and major findings
| Study | ROB | Sample | MS Type | Intervention | Timeline | Duration | Major Findings |
|---|---|---|---|---|---|---|---|
| Kouchaki et al.60 | Low | 18–55 yo n = 30/g |
RRMS EDSS ≤ 4.5 |
T: Probiotic capsule, 2 × 109 CFU (L. acidophilus, L. casei, B. bifidum, L. fermentum) C: starch capsule |
Thera | Biweekly for 12 wks |
CD: ↓ EDSS, BDI, GHQ-28, DASS IM: ↓ hs-CRP, MDA; ↑ plasma NO MM: NA MC: NA |
| Rahimlou et al.63 | Low | 18–50 yo n = 35/g |
RRMS EDSS ≤ 4.5 |
T: “Protexin”a capsule, 2 × 109 CFU C: maltodextrin capsule |
Thera | Daily for 6 mos |
CD: – EDSS; ↓ BDI, GHQ-28, FSS & MPQ IM: ↓ IL-6; ↑ BDNF; – NGF MM: NA MC: NA |
| Salami et al.64 | Low | 20–60 yo n = 24/g |
RRMS EDSS ≤ 4.5 |
T: Probiotic capsule, 2 × 109 CFU (L. plantarum, L. casei, L. reuteri, L. fermentum, B. lactis, B. infantis) C: maltodextrin capsule |
Thera | 16 wks (freq. not specified) |
CD: ↓ EDSS, DASS; – BDI, GHQ-28 IM: ↓ IL-6, TNF-α, hs-CRP, MDA, 8-OHdG; ↑ IL-10, TAC, GSH, NO MM: NA MC: NA |
| Tamtaji et al.61 | Low | 18–55 yo n = 20/g |
RRMS EDSS ≤ 4.5 |
T: Probiotic capsule, 2 × 109 CFU (L. acidophilus, L. casei, L. fermentum, B. bifidum) C: starch capsule |
Thera | Daily for 12 wks |
CD: – BMI; no relapses IM: ↓ IL-8, TNF-α; – IL-1, PPAR-γ, LDLR MM: NA MC: NA |
| Tankou et al.46 | High |
n = 7 MS, taking GA n = 2 MS, untreated n = 13 HC |
RRMS |
T: VSL3,b 3.6 × 1012 CFU/d HC: VSL3,b 3.6 × 1012 CFU/d |
Thera | Daily for 2 mos |
CD: NA IM: ↑ freq. IL-10+ Tregs; ↓ freq. intermediate & inflammatory monocytes, costimulatory marker CD80 on classical monocytes, MFI of HLA-DR on myeloid-derived DCs, rel freq. of TH1 & TH17 cells; ↓ freq. IL-10+ Tregs & ↑ freq. of inflammatory monocytes following cessation; – freq. B cells, NK cells, myeloid or plasmacytoid DCs, naïve CD4 or CD8 T cells, central memory CD4 or CD8 T cells, effector memory CD4 T cells MM: ↑ rel. abund. of Lactobacillus, Streptococcus, & Bifidobacterium, which returned to baseline following cessation MC: NA |
| Tankou et al.47 | High |
n = 7 MS, taking GA n = 2 MS, untreated n = 13 HC |
RRMS EDSS = 1.4 ± 0.9 |
T: LBS,c 3.6 × 1012 CFU/d HC: LBS,c 3.6 × 1012 CFU/d |
Thera | Daily for 2 mos |
CD: NA IM: ↑ freq. IL-10+ Tregs; ↓ freq. intermediate & inflammatory monocytes, costimulatory marker CD80 on classical monocytes, MFI of HLA-DR on myeloid-derived DCs, rel freq. of TH1 & TH17 cells; ↓ freq. IL-10+ Tregs & ↑ freq. of inflammatory monocytes following cessation; – freq. B cells, NK cells, myeloid or plasmacytoid DCs, naïve CD4 or CD8 T cells, central memory CD4 or CD8 T cells, effector memory CD4 T cells MM: ↑ rel. abund. of Lactobacillus, Streptococcus, & Bifidobacterium; ↑ rel. abund. of Veillonellaceae family & Collinsela genus; ↓ rel. abund. of Akkermansia, Blautia, Dorea, B. adolescentis; microbiome changes returned to baseline following cessation & were associated with changes in stool metabolic profile MC: ↓ expression of MS risk allele HLA.DQA.1, & HLA.DPA1, ILGST, MALT1, LGALS3 in monocytes; ↑ expression of IL-10RA, LILRB2, CYBB; most returned to baseline following cessation. |
All findings are reported with respect to control group(s) unless otherwise indicated.
aProtexin = B. subtilis PXN 21, B. bifidum PXN 23, B. breve PXN 25, B. infantis PXN 27, B. longum PXN 30, L. acidophilus PXN 35, L. rhamnosus PXN 54, L. helveticus PXN 45, L. salivarius PXN 57, L. lactis ssp. lactis PXN 63, S. thermophilus PXN 66, L. casei PXN 37, L. delbrueckii ssp. bulgaricus PXN 39, L. plantarum PXN 47.
bVSL3 = L. paracasei DSM 24732, L. plantarum DSM 24730, L. acidophilus DSM 24734, L. delbruckeii subsp. bulgaricus DSM 24734, B. longum DSM 24736, B. infantis DSM 24737, B. breve DSM 24732, S. thermophilus DSM 24731.
cLBS = see bVSL3.
Key: ↓ decreased; ↑ increased; – no change or no difference compared to control; NA, not applicable to this study.
Abbreviations: ROB, risk of bias; MS, multiple sclerosis; yo, y old; g, group; RRMS, relapsing-remitting multiple sclerosis; GA, glatiramer acetate; HC, healthy controls; EDSS, Expanded Disability Status Scale; T, treatment group; C, control; CFU, colony forming units; Thera, therapeutic; wks, weeks; mos, months; freq., frequency; CD, clinical disease; IM, immune/metabolic; MM, microbiome/metabolome; MC, mechanistic/correlative; BDI, Beck Depression Inventory; GHQ-28, General Health Questionnaire-28; DASS, Depression Anxiety Stress Scales; hs-CRP, high-sensitivity C-reactive protein; MDA, malondialdehyde; NO, nitric oxide; FSS, Fatigue Severity Scale; MPQ, McGill Pain Questionnaire; IL, interleukin; BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor; TNF-α, tumor necrosis factor alpha; 8-OHdG, 8-Oxo-2ʹ-deoxyguanosine; TAC, total antioxidant capacity; GSH, glutathione; BMI, body mass index; HLA-DR, human leukocyte antigen-antigen D related; DC, dendritic cell; rel. abund., relative abundance; NK, natural killer.
Table 3.
Animal studies of probiotic therapies: summary of study characteristics and major findings
| Study | ROB | Sample | MS Model | Intervention | Timeline | Duration | Major Findings |
|---|---|---|---|---|---|---|---|
| Maassen et al.59 | UN | F SJL mice 8–12 wko n = 6/g |
PLP-induced EAE |
T1: L. reuteri ML1 T2: L. casei 393 T3: L. plantarum NCIB 8826 T4: L. murinus CNRZ Each 1010 CFU o.g. C: NaCO3 |
Proph | Every other day for 5 admins |
CD: ↑ CDB (T1), weak ↓ (T2), ↓ (T4), – (T3) IM: NA MM: NA MC: NA |
| Salehipour et al.50 | Low | F C57BL6 mice 8–10 wko n = 8/g |
MOG-induced EAE |
T1: L. plantarum A7, 109 CFU o.g. T2: B. animalis PTCC 1631,109 CFU o.g. T3: T1+ T2 C: sterile saline |
Thera | Daily for 22 d |
CD: delayed EAE onset (T1-T3), T3 more pronounced; ↓ EAE CS, CDI, incidence, infiltration of MNCs, and demyelination (T1-T3), T3 more pronounced IM: ↓ ASP, IFN-γ, IL-6, IL-17 (T1-T3), T3 more pronounced; ↑ % CD4+ CD25+ Foxp3+ Tregs, IL-4, IL-10, TGF-β (T1-T3), T3 more pronounced MM: NA MC: ↑ expression of GATA3, FoxP3 and ↓ expression of Tbet, RORγt (T1-T3), T3 more pronounced |
| He et al.75 | UN | F C57BL6 mice 10–12 wko n = 37–40/g |
MOG-induced EAE |
T: L. reuteri DSM 17938 100 µL of 108 CFU o.g. C1: 100 µL MRS media + EAE, o.g. C2: normal control |
Thera | Daily for 20 d |
CD: ↓ CDS, EAE incidence, maximum CS, inflammatory cell infiltration IM: ↓ CD3 + T cells & CD68+ macrophages in spinal cord, % and abs. # of TH1 and TH17 cells, IL-17, IFN-γ, ↓ % MOG35-55-specific splenocytes MM: reversed EAE rel. abund. changes (↑ Bacteriodetes, Proteobacteria, Deferribacteres); Bifidobacterium, Lactobacillus, Prevotella, & S24-7 = negative correlation w/ CS MC: NA |
| Goudarzvand et al.52 | UN | M Wistar rats 8–10 wko n = 8/g |
GID |
T1: L. plantarum T2: Bifidobacterium B94 Each 1.5 × 108 CFU/mL orally C1: GID only C2: sterile saline, no GID |
Thera | Daily for 28 d |
CD: – traveled distance, escape latency, or swimming speed IM: NA MM: NA MC: NA |
| Consonni et al.53 | Low | F Lewis rats 6–8 wko n = 6–9/g (T1-T4), n = 18/g (T5-T6) |
gpMBP-induced EAE |
T1: L. crispatus LMG P-23257 T2: L. rhamnosus ATCC 53103 T3: B. animalis subsp. lactis BB12 T4: B. animalis subsp. LMG S-28195 T5: T1+ T2 T6: T3+ T4 Each 2 × 109 CFU/300 µL orally C: vehicle |
Proph & Thera | 15 doses over 3 wks (5 doses pre-EAE) |
CD: ↓ EAE incidence & median score at peak (T1-T6); ↓ myelin loss, astrocytosis, & spinal cord immune cell infiltration (T5/T6); delayed EAE onset (T1,T2, T5, T6); dose-response relationship observed with T5/T6 IM: ↓ proliferative response to MBP, IFN-γ, TNF-α, IL-17 (T5/T6); ↑ TGF-β, IL-16 (T5/T6) MM: NA MC: NA |
| Baken et al.73 | UN | M Lewis rats 6–8 wko n = 8/g |
gpSCH-induced EAE |
T: L. casei strain Shirota, 1 mL of 1 × 109 CFU/mL C: 1 mL saline/peptone |
Proph & Thera | Daily for 35 d (starting 8 d pre-EAE) |
CD: ↓ body weight; ↑ EAE incidence, duration, CDS, & CDI; earlier EAE onset IM: NA MM: NA MC: NA |
| Gharehkhani Digehsara et al.71 | UN | F C57BL6 mice 8–10 wko |
Cuprizone (4 wks) |
T1: L. casei 4 wks, cuprizone 4 wks T2: Cuprizone 4 wks, L. casei 4 wks T3: Cuprizone 4 wks, L. casei 4 wks w/ Vitamin D3 (20 IU/d) C1: L. casei 4 wks, 1 × 109 CFU/mL C2: Cuprizone 4 wks, 0.2% w/w All admin orally C3: normal control |
Proph & Thera |
CD: more normal & significant Y-maze alternation behavior (T1-T3) IM: ↓ IL-17 (T1-T3), T3 more pronounced; ↑ TGF-β (T1-T3) MM: NA MC: ↓ expression of IDO gene & miR-155 (T1-T3, C2); ↑ expression of miR-25 (C2), trending in T1-T3 |
|
| Kobayashi et al.68 | UN | M & F Lewis rats 7 wko & 2 wko n = 8/g |
gpSCH- or gpMBP-induced EAE |
T1: L. casei strain Shirota in M rats (7 wko), 1–2 × 109 CFU o.g. T2: L. casei strain Shirota in F rats (7 wko), 1–2 × 109 CFU o.g. T3: L. casei strain Shirota in M & F rats (2 wko), 9.2–10.1 × 109 CFU o.g. T4: B. breve strain Yakult in M & F rats (2 wko), 5.0–6.9 × 109 CFU o.g. C: 0.5 mL saline/peptone |
Proph & Thera | Daily T1-T2: 35 d (starting 7 d pre-EAE) T3-T4: 63 d (starting 5 wks pre-EAE) |
CD: ↓ mortality in T1-T4 except T4 males (↑); – EAE onset, peak, mean CDS, infiltration of MNCs IM: – MBP IgG MM: NA MC: NA |
| Kobayashi et al.74 | UN | F SJL & C57BL6 mice 7 wko n = 15/g |
PLP-induced EAE (SJL) & MOG-induced EAE (C57BL6) |
T: L. casei YIT 9029, 0.6–1.2 × 109 CFU o.g. C: 0.2 mL saline/peptone |
Proph & Thera | Daily for 50 (SJL) or 29 (C57BL6) d (both starting 1 wk pre-EAE) |
CD: ↓ EAE CS on ds 12, 29, & 30 (SJL); – EAE onset, peak score, MNC infiltration, white matter demyelination, or neutrophil infiltration (SJL & C57BL6) IM: ↓ % CD8 + T cells in spleen (SJL), ↑ IL-10, % Tregs in spleen, IL-17, IFN-γ (SJL) MM: NA MC: NA |
| Lavasani et al.48 | UN | F C57BL6 (WT & IL-10-/-) mice 8–10 wko n = 3–18/g |
MOG-induced EAE |
T1: Proph L. paracasei DSM 13434 T2: Proph L. plantarum DSM 15312 T3: Proph L. plantarum DSM 15313 T4: Proph L. paracasei PCC 101 T5: Proph L. delbrueckii subsp. bulgaricus DSM 20081 Each 5 mL of 109 CFU orally until EAE, then 200 µL of 109 CFU o.g. T6: Thera T1 T7: Thera Lacto-mix (T1-T3) T8: HK T7 T9: T7 in IL-10-/- mice Each 200 µL of 109 CFU o.g. C: saline |
Proph & Thera | Daily for 37 d (starting 12 d pre-EAE) or every other day for 20 d (starting 2 wks post-EAE onset) |
CD: – EAE progression (T1-T5,T8); delayed EAE onset and ↓ CS (T1-T3, T7); therapeutic effects of Lacto-mix absent in T9 IM: ↓ T cell proliferation (T1-T3) ↓ CD4 + T cells (T1,T3),, ↓ IFN-γ, TNF-α (T1); ↑ IL-4, IL-10, TGF-β (T1); ↓ CNS inflammation, CD4 + T cell infiltration, IFN-γ, TNF-α, IL-17, and IL-17-producing CD4 + T cells (T7); ↑ IL-10, IL-10-producing CD4 + T cells, Tregs, Foxp3+ cells in brain, mLN, spleen (T7) MM: NA MC: EAE suppressed in recipient mice (mLN cells from T7) & depletion of CD4+ CD25 + T cells from mLN cells reverses suppression |
| Sanchez et al.49 | UN | M & F C57BL6 (WT, CD45.1, CD45.2, GF, TLR-2-/-, & TLR-9-/-) mice & SJL mice 9–10 wko n ≥ 10/g |
MOG-induced EAE (C57BL6) & PLP-induced EAE (SJL) |
T1: Proph L. paracasei ATCC 27092 in C57BL6 mice T2: Proph HK T1 in C57BL6 mice T3: Proph L. paracasei DSM 2649 in C57BL6 mice T4: Proph L. paracasei ATCC 11582 in C57BL6 mice T5: Proph L. paracasei ATCC 334 in C57BL6 mice T6: Proph L. paracasei DSM 5622 in C57BL6 mice T7: T2 in CD45.1 C57BL6 donor mice AT into CD45.2 C57BL6 mice T8: T2 in TLR-2-/- C57BL6 mice T9: T2 in TLR-9-/- C57BL6 mice T10: T2 in WT C57BL6 mice GMT into GF C57BL6 mice T11: Thera T2 in SJL mice Each 109 CFU o.g. C: PBS, MRS medium, or T1-conditioned medium |
Proph & Thera | Daily for 34–39 d (starting 2 wks pre-EAE) or daily for 39 d (starting 21 dpi) T10: daily for 28 d (starting 70 d pre-EAE) and GMT at 42 d pre-EAE |
CD: ↓ EAE CS (T1-T6); ↓ EAE incidence (T1-T6); ↓ demyelination, infiltrating macrophages & lymphocytes in brain & spinal cord, and severity of subsequent relapses (T1-T2); ↑ EAE CS w/ T1-conditioned media; – BBB & BSCB permeability (T2) IM: – prop. CD4+ IFN-γ + T cells, CD4+ IL-17A+ T cells, or Tregs in CNS (T1-T2); ↓ CCL3, CCL4, CXCL5, CXCL13 (T2) MM: ↓ EAE incidence (T10); – EAE CS (T10) MC: EAE suppression and ↓ demyelination eliminated in TLR2-/- mice; – EAE incidence or severity in AT recipient mice of T2 |
| Yamashita et al.67 | UN | F SJL mice 5 wko n = 10/g |
PLP-induced EAE |
T: HK L. helveticus SBT2171, 1 mg i.p. C1: 1 mg PBS i.p. C2: PBS, no EAE (n = 3) |
Proph & Thera | 3×/wk for 3 wks (pre-EAE), daily for 42 dpi |
CD: ↓ EAE incidence, CS, enlargement of inguinal LNs, infiltrating MNCs IM: ↓ # TH17, TH1, & CD4 + T cells in spinal cord, IL-17; ↓ IL-6, TGF-β, Foxp3+, IFN-γ in inguinal LNs; – IL-10 in inguinal LNs, – Ccl20 in spinal cord, CCR2, CCR4, & CCR6 on TH17 cells in dLNs MM: NA MC: NA |
| Libbey et al.54 | UN | M C57BL6 mice 4 wko n = 15/g |
MOG-induced EAE |
T1: rE. coli Nissle 1917, 1.31 × 109 CFU o.g. single gavage day T2: rE. coli Nissle 1917, 1.19 × 109 CFU + 1.25 × 109 CFU o.g. double gavage C1: 70 µL PBS C2: rE. coli Nissle 1917, no EAE |
Proph | 1–2 admins 3 or 7 d pre-EAE Follow for 35–36 dpi |
CD: ↓ survival & – CS, onset, incidence, CDS, maximal score, meningitis, demyelination, PVC (T1); ↓ CS, weight loss, PVC in T2; ↑ survival (T2); – EAE onset, incidence, CDS, maximal score, meningitis, demyelination (T2) IM: ↑ microglia, Tregs, IFN-γ, IL-27 (T1); ↓ CNS-derived cells, microglia, CD8 + T cells, CD4 + T cells, TH1 cells, Tregs in brain (T2); ↑ TH17 cells (T2) MM: NA MC: NA |
| Secher et al.55 | UN | M C57BL6 mice 8–12 wko n = 30–40/g |
MOG-induced EAE |
T1: E. coli Nissle 1917 T2: archetypal K12 E. coli MG1655 Each 108 CFU o.g. C: PBS |
Proph & Thera | Daily for 37 d (starting 7 d pre-EAE) |
CD: ↓ EAE CS, mortality, incidence, CDS, maximal score; – onset; ↓ colon & ileum permeability IM: ↓ total CD4+ and MOG-specific CD4 + T cells in spinal cord, IFN-γ, GM-CSF, IL-17, TNF-α, IL-6; ↑ total CD4+ and MOG-specific CD4 + T cells in LNs, IL-10, CD4+ Foxp3+ cells from draining LNs MM: NA MC: ↑ expression of Reg3g, Reg3b, Claudin-8, ZO-1 |
| Mestre et al.43 | UN | F SJL mice 5–8 wko n = 5–10/g |
TMEV-IDD |
T: TMEV-IDD w/ Vivomixxb 100 µL of 3 × 108 CFU o.g. C1: Sham w/ Vivomixxb C2: TMEV-IDD w/ vehicle C3: Sham w/ vehicle |
Thera | 3×/wk for 15 d (70–85 dpi) |
CD: ↑ horizontal & vertical activity, latency to fall IM: ↓ CD4 + T cells, B cells, % Foxp3+ CD39+ and Foxp3-CD39 + T cells in spleen, IL-1B, IL-6; ↑ Bregs, IL-10; – TNF-α; microglia exhibited anti-inflammatory activation or ↓ proinflammatory activity MM: ↓ rel. abund. of Anaerostipes, Dorea, Oscillospira, Enterobacteraceae, Ruminococcus, Bilophila, ↑ rel. abund. of Bacteroides, Odoribacter, Lactobacillus, Sutterella; ↑ acetate & butyrate in plasma MC: NA |
| Calvo-Barreiro et al.44 | Low | F C57BL6 OlaHsd mice 8 wko n = 17–20/g |
MOG-induced EAE |
T1: Lactibiane ikic, 1.6 × 109 CFU o.g. once daily T2: T1 twice daily T3: Vivomixxb, 9 × 109 CFU o.g. once daily T4: T3 twice daily C1/C2: water o.g. once or twice daily C3: untreated EAE C4: normal control |
Thera | 1–2× daily for 18–22 d (13–16 or 12–15 dpi) |
CD: ↓ CS w/ T1/T2 but not T3/T4 (dose-response observed); ↓ % demyelination, T cell inflammatory infiltrate density, & axonal damage (T1-T4); – intestinal permeability or microglia or astrocyte reactivity; improved motor coordination IM: ↓ ASP w/ T1/T2 but not T3/T4, % peripheral plasma cells (T1/T2); ↑ Tregs (T1/T2) MM: – alpha or beta diversity; ↑ rel. abund. of Lachnoclostridium and Bifidobacterium (T1/T2), Streptococcus (T3/T4); Atopobiacaeae & Bifidobacterium assoc. with ↓ accumulated EAE scores MC: 4x↓ expression of Th17 txn factor RORγt in spinal cord T1/T2 |
| Kwon et al.69 | UN | C57BL6 mice 6–8 wko n = 10/g |
MOG-induced EAE |
T1: Proph IRT5d T2: Thera IRT5d Each 5 × 108 CFU o.g. C: PBS |
Proph & Thera | Daily for 3 wks (pre-EAE) or 16 d (starting 12 dpi) |
CD: ↓ EAE incidence, CS, lymphocytes, Gr1+ and CD11b+ monocytes, and CD4 + T cells in spinal cord (T1); delayed EAE onset & ↓ EAE CS (T2) IM: ↓ ASP, IFN-γ, TNF-α, IL-17; ↑ IL-4, IL-10, CD4+ Foxp3+ Tregs from spinal cord MM: NA MC: NA |
| McMurran et al.45 | Low | F C57BL6 mice 13 mo n = 3–5/g |
Cuprizone (5 wks) |
T: VSL#3e, 1.35 × 109 CFU o.g. C: autoclaved water |
Proph & Thera | Daily for 7 wks (starting 4 wks pre-lysolecithin injection) |
CD: – remyelination IM: ↑ SCFAs in feces & serum, inflammatory response at 5 dpi, density of CD68+ activated microglia & infiltrating macrophages, # of ODCs at 5 dpi; – ODC response at 14 dpi, inflammatory response at 14 dpi MM: NA MC: NA |
| Ezendam et al.51 | UN | M & F Lewis rats 2 wko n = 4–8/g |
gpMBP-induced EAE |
T: B. animalis w/ EAE, 200 µL of 1 × 109 CFU o.g. C1: B. animalis w/ no EAE C2: 200 µL saline w/ EAE C3: 200 µL saline w/ no EAE |
Proph & Thera | Daily for ~60 d (starting 5 wks pre-EAE) |
CD: ↑ weight gain for M; shorter EAE duration for M; – EAE onset, duration, or CDI for F; – EAE onset or CDI for M IM: NA MM: NA MC: NA |
| Ezendam & van Loveren72 | High | M & F Lewis rats 2 wko n = 4–8/g |
gpMBP-induced EAE |
T; L. casei strain Shirota, 500 µL of 1–2 × 109 CFU o.g. C: 500 µL saline/peptone |
Proph & Thera | Daily for ~9 wks (starting 5 wks pre-EAE) |
CD: – EAE onset, CS, CDI for M & F; slightly longer duration for F; ↑ EAE incidence IM: NA MM: NA MC: NA |
| Johanson et al.76 | UN | F C57BL6 mice 8 wko n = 10/g |
MOG-induced EAE |
T: L. reuteri ATCC 2327 ad libitum C: MRS broth |
Thera | Daily for 20 d |
CD: ↓ average CS, – weight loss IM: NA MM: ↑ Lactobacillus 16S V3-V4 amplicon abundance MC: NA |
| Abdurasulova et al.66 | UN | F Wistar rats 3 mo n = 26–35/g |
homologous SCH-induced EAE |
T1: E. faecium LMG P-27496 L3 probe, 0.5 mL o.g. T2: Glatiramer acetate, 0.2 mL s.c. C1: saline, 0.5 mL o.g. C2: saline, 0.2 mL s.c. |
Thera | Daily for 15 d (starting 2 dpi) |
CD: ↓ prevalence, CDS, CS, mortality (T1/T2); delayed onset and shorter duration (T1/T2); T1 outperformed T2 in prevalence, duration, and CDS IM: ↓ CD4+ CD25+ Foxp3+ Tregs during peak and recovery phases, CD4 + T cells during peak; ↑ CD4+ Tcells during inductive, CD4+ CD25+ Foxp3- Tregs during peak, CD8 + T cells during peak & recovery MM: NA MC: NA |
All findings are reported with respect to control group(s) unless otherwise indicated.
bVivomixx = L. paracasei DSM 24734, L. plantarum DSM 24730, L. acidophilus DSM 24735, L. delbrueckii subsp. bulgaricus DSM 24734, B. longum DSM 24736, B. infantis DSM 24737, B. breve DSM 24732, S. thermophilus DSM 24731.
cLactibiane iki = B. lactis LA 304, L. acidophilus LA 201, L. salivarius LA 302.
dIRT5 = L. casei, L. acidophilus, L. reuteri, B. bifidum, S. thermophilus.
eVSL#3 = see bVivomixx.
Key: ↓ decreased; ↑ increased; – no change or no difference compared to control; NA, not applicable to this study.
Abbreviations: ROB, risk of bias; MS, multiple sclerosis; UN, uncertain; M, male; F, female; wk/wks/wko, week/weeks/weeks old; mo/mos, month/months; g, group; WT, wild-type; IL, interleukin; GF, germ-free; TLR, toll-like receptor; EAE, experimental autoimmune encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein; PLP, proteolipid protein; MBP, myelin basic protein; gp, guinea pig; SCH, spinal cord homogenate; GID, gliotoxin-induced demyelination; TMEV-IDD, Theiler’s murine encephalomyelitis virus-induced demyelinating disease; CFU, colony-forming units; T, treatment group; C, control; o.g., oral gavage; IU, international units; w/w, weight/weight; HK, heat-killed; -/-, deficient; AT, adoptive transfer; GMT, gut microbiome transfer; i.p., intraperitoneally; PBS, phosphate-buffered saline; Proph, prophylactic; Thera, therapeutic; admins, administrations; dpi, days post immunization; CD, clinical disease; IM, immune/metabolic; MM, microbiome/metabolome; MC, mechanistic/correlative; CDB, clinical disease burden; CS, clinical score; CDI, clinical disease index; MNC, mononuclear cell; IgG, immunoglobulin G; ASP, antigen-specific proliferation; IFN-γ, interferon gamma; TGF-β, transforming growth factor beta; CDS, cumulative disease score; rel. abund., relative abundance; TNF-α, tumor necrosis factor alpha; CNS, central nervous system; LN, lymph node; BBB, blood–brain barrier; BSCB, blood spinal cord barrier; PVC, perivascular cuffing; GM-CSF, granulocyte monocyte colony-stimulating factor; txn, transcription; SCFA, short-chain fatty acid; ODC, oligodendrocyte; abs., absolute
Figure 1.
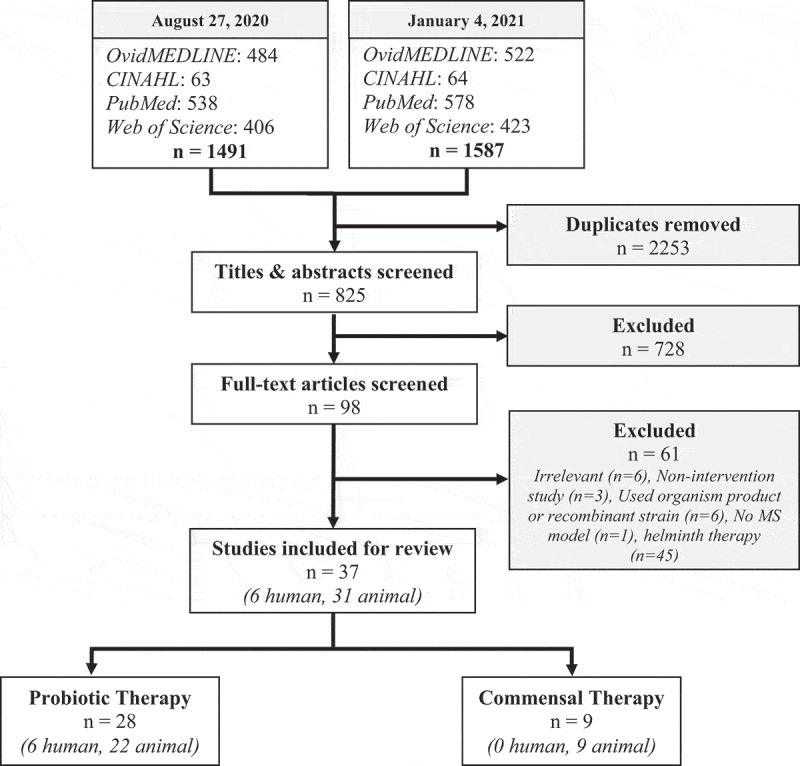
Search strategies, selection criteria, and inclusion of articles. Articles from four separate databases were identified and screened for inclusion. Note that helminth therapy studies were included in the search strategy but manually removed during the eligibility stage of the second search
Risk of bias
Overall, 10 studies (4 human, 6 animal) were deemed “high quality” based on the low risk of bias determined using the Cochrane ROB and SYRCLE tools (Supp. File 1D and 1 F). Most (n = 24) of the studies (0 human, 24 animal) were classified as “medium quality” due to study design limitations or failure to disclose randomization and/or blinding efforts. The remaining studies (n = 3) were classified as “low quality,” including two human studies that had important baseline characteristic differences between groups, substantial risk of confounding due to concurrent use of a DMT (glatiramer acetate), and compared the results to healthy controls rather than untreated MS patient controls46,47,; and one animal study that was not powered to perform statistical analysis lacked clarity regarding the control groups and did not explicitly state the use of randomization or blinding measures.72
Study design
Four human studies were structured as double-blind, placebo-controlled RCTs,60,61,63,64 and the remaining two were prospective cohort studies.46,47, The animal models of MS included EAE (n = 27), cuprizone-45,71 or gliotoxin-induced demyelination,52 and Theiler’s murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD).43
Subjects
Human subjects were studied exclusively in the RRMS stage. Expanded disability status scores (EDSS) were all ≤4.5 as a maximum for inclusion. Male and female participants ranged from 18 to 60 y old. Animal subjects included both male and female rats (Lewis and Wistar) and mice (C57BL/6, SJL, and PWD/PhJ) ranging in age from 2 weeks to 13 months, with a mean of 7–8 weeks old. Various genetically modified C57BL/6 mice were used, including HLA-DR3.DQ8 transgenic56,57,58 and IL-10-,48 TLR-2-,49 and TLR-9-49 deficient strains.
Interventions
Probiotic therapy involved individual strains and various combinations (including Lacto-mix, Lactibiane iki, IRT5, Protexin, VSL3/VSL#3/Vivomixx/LBS) of Lactobacillus spp. including L. casei, L. paracasei, L. plantarum, L. reuteri, L. delbrueckii subsp. bulgaricus, L. crispatus, L. rhamnosus, L. murinus, L. brevis, L. helveticus, L. lactis subsp. lactis, L. fermentum, L. acidophilus, and L. salivarius, Bifidobactererium ssp. including B. animalis, B. B94, B. breve, B. longum, B. bifidum, B. infantis, B. lactis, B. subtilis as well as E. coli Nissle 1917, E. faecium, and S. thermophilus. All four human RCT studies utilized probiotic combinations at 2 × 109 CFU/mL ranging from 12 weeks to 6 months duration, while the prospective cohorts used 3.6 × 1012 CFU/d for 2 months. The animal studies administered a mean of 109 CFU/mL (range of 108–1010 CFU/mL) for 2–7 weeks duration. Probiotics were administered prophylactically,54,59, therapeutically,43,44,46,47,50,52,60,61,63,64,66,75,76 or both.45,51,55,69,72,
There were no human studies using commensal therapy identified. Animal studies used Prevotella histicola (P. histicola),56,57,58 P. acidilactici,70C. butyricum,65Akkermansia muciniphila (A. muciniphila),35 L. reuteri37,38, (by stable colonization), Allobaculum,38 and B. fragilis62 at a dosage ranging 5 × 106 to 1 × 1010 CFU/mL for one administration or 2–5 weeks. Commensals were administered prophylactically,37,38,62,65 therapeutically,35 or both.56,57,58,70
Measurements and outcomes
The most common measurements for clinical parameters for human studies included EDSS, mental health and quality of life assessments (Beck Depression Inventory (BDI), General Health Questionnaire-28 (GHQ-28), Depression Anxiety Stress Scale (DASS), Fatigue Severity Scale (FSS), McGill Pain Questionnaire (MPQ)). Notably, none of the human studies assessed MRI lesions. For animal studies, clinical signs of motor disability and associated quantitative variables (EAE incidence, onset, duration, and clinical scores; motor function, coordination, and activity for other MS models), histopathology (demyelination, CNS infiltration), BBB and intestinal permeability, and weight loss. Cytokine analysis, oxidative stress/antioxidant markers, and immunophenotyping were the most commonly measured immune/metabolic indices. Microbiome and metabolome assessments were primarily measured using fecal microbiome analysis, and fecal/serum short-chain fatty acid (SCFA) production. Gene expression and adoptive transfer experiments comprised additional mechanistic and correlative findings.
B. Probiotic therapy
Major trends
The major findings of each probiotic therapy study included can be found in Tables 2 and 3 for human and animal studies, respectively. A qualitative summary of these studies is provided below, followed by a semi-quantiative ranked evaluation using BH criteria.
Clinical studies
Four of the human probiotic therapy studies included were double-blind, placebo-controlled RCTs, and thus all were classified as “high quality” studies.60,61,63,64 Probiotic therapy produced modest decreases in EDSS that, while sometimes statistically significant, were not found to be clinically significant based on the authors’ designation of an EDSS change of ≥1.0 point for levels less than 5.5 or ≥0.5 point for levels greater than 5.5.60,64 The impact on EDSS seemed more pronounced in the shorter, 12 week study, suggesting that the observed benefits may only be transient.60 Probiotic therapy did, however, lead to marked improvements in quality of life as measured through the BDI, GHQ-28, DASS, FSS, and MPQ assessments.60,63,64 The proinflammatory cytokines that were measured (IL-6, IL-8, and TNFα) were consistently reduced in the probiotic treatment groups, as were several oxidative stress markers (hs-CRP, MDA).60,61,63,64 Anti-inflammatory cytokines and antioxidants were measured, showing elevated IL-10 and plasma nitric oxide.60,64
Two additional prospective cohort studies used therapy with the probiotic mixture VSL#3.46,47 While neither study focused on clinical outcome, both found that VSL#3 elicited changes in the peripheral immune response consistent with an immune regulatory state, including phenotypic changes in monocytes and dendritic cells and decreased expression of the MS risk allele HLA-DQA1. Additionally, these studies found an increased relative abundance of Lactobacillus, Bifidobacterium, Streptococcus spp. in stool, which is consistent with the species that were administered in probiotic form in the VSL#3 formulation. Further, one study found an increased relative abundance of Collinsela and Veillonellaceae family members that are typically depleted in MS gut microbiomes, as well as a decreased relative abundance of Akkermansia, Blautia, and Dorea genera, which are typically enriched.46 Notably, these differences display an inverse relationship in MS patient cohort gut microbiomes, suggesting that VSL#3 may restore the MS-dysbiotic state.
Preclinical studies
The majority (26 out of 30) of animal studies used the EAE model. This is an important consideration, since it is a model driven by an autoimmune response, thus immunomodulation is the most likely mode of action for any effects on clinical disease. More studies investigated the effects of Lactobacillus spp.48–50,52,53,59,67,68,71–76 and probiotic combinations43–45,48,50,53,69 rather than Bifidobacterium spp.51–53,68 and E. coli Nissle 1917,54,55 and just a single study utilized E. faecium.66
Proportionally, Lactobacillus spp. tended to outperform Bifidobacterium spp. in reducing EAE incidence, onset, clinical score, duration, demyelination, immune cell infiltration, and motor activity. Positive clinical outcomes were observed in about 64% of Lactobacillus spp. studies (n = 14), 25% of Bifidobacterium spp. studies (n = 4), 100% of E. coli Nissle 1917 studies (n = 2), and about 81% of probiotic combination studies (n= 11), while E. faecium was shown to be at least as effective as the standard MS DMT glatiramer acetate.66 L. casei Shirota,68,72,73 and B. animalis50–53 were the least clinically successful therapies among the studies investigated, while L. paracasei,48,49 L. plantarum,48,50 and E. coli Nissle 191754,55 appeared the most successful. Notably, despite disparate outcomes, VSL#345 and Vivomixx43,44 contain the same probiotic formulation, although importantly each study used a different model: cuprizone-induced demyelination/remyelination vs. TMEV-IDD vs. EAE, respectively, with the former (cuprizone) lacking a strong immune-mediated component. Furthermore, L. paracasei49 and the combinations of L. crispatus and L. rhamnosus53 and B. animalis subsp. lactis strains53 were able to elicit clinical benefits whether administered live or heat-killed, while Lacto-mix48 was only effective when live probiotic organisms were used. Five studies demonstrated a dose–response relationship,44,48,50,53,54 and three studies provided evidence for the combinatorial effects of probiotics.43,45,69 Only ~32% of the animal studies (n = 22) reported primarily no effect or exacerbation of clinical disease.51,59,68,72–75 It should also be noted that only four of the studies were classified as high quality, all of which reported positive results with probiotics.44,45,50,53
The majority of studies reported favorable secondary immunological findings, with elevated levels of anti-inflammatory cytokines (IL-10, IL-4, TGF-β)43,48,50,53,55,59,69,71,74 and CD4+ CD25+ FoxP3+ Tregs44,50,54,55,69,74 and reduced levels of proinflammatory cytokines (IL-17, IL-1, IL-6, TNF-α, IFN-γ),43,48,50,53,55,67,69,71,75 chemokines (CCL3, CCL4, CXCL5, CXCL13),49 and TH1 and TH17 cells.43,54,55,67,69,74,75 Multiple Lactobacillus spp. and probiotic combinations also demonstrated decreased antigen-specific T cell proliferation.44,48,50,53,69 Putatively beneficial microbiome changes included an increased relative abundance of Firmicutes, Bacteriodetes, Proteobacteria phyla and Sutterella, Bifidobacterium, Streptococcus, Lactobacillus, and Prevotella spp.43,44,75 Two studies measured SCFA production and found increased levels in both serum and feces.43,45 Four studies reported increased expression of TH2 and Treg regulators (GATA3, Foxp3),50 miR-25,71 antimicrobial peptides (Reg3g, Reg3b),55 and tight junction proteins (Claudin-8, ZO-I);55 and decreased expression of TH1 and TH17 regulators (Tbet, RORγt),44,50 miR-155,71 and the IDO gene,71 a potential marker of MS/EAE relapses. Furthermore, Lavasani et al. performed an adoptive transfer experiment of CD4+ CD25 + T cells from mesenteric lymph nodes of the probiotic Lacto-mix group and found that the recipient mice had suppressed EAE symptoms and elevated IL-10 levels.48 These effects, however, were eliminated when tested in IL-10-deficient mice.48 Notably, Sanchez et al. also included an adoptive transfer experiment of splenic and mesenteric lymph node leukocytes of heat-killed L. paracasei–treated donor mice into recipient mice and found no such effects.49 Additional mechanistic findings included decreased intestinal barrier permeability55 and oligodendrocyte differentiation enhancement.45
BHC scores and rankings
The BH score calculations and findings for probiotic therapy are detailed in Supplemental File 2, and summarized in Table 5. Given the large number of studies and treatments, we do not discuss each individually, but instead highlight and contrast some of the key findings below.
One probiotic treatment approach emerged as the most strongly supported (BH score = 9), namely the VSL#3 multi-species formulation, which was assessed in two human and three animal studies. Two out of three animal studies reported significant clinical improvement in the EAE model. The third study used the cuprizone demyelination model and reported a lack of clinical improvement, but some favorable histologic changes. The human studies did not measure clinical parameters, but reported immunological and microbiological changes that would be consistent with favorable immune modulation. Hence, this particular approach satisfied seven out of eight BH criteria (BHC #1, 3–8), with high evidence for replication (Table 5). In contrast, another combination treatment (L. acidophilus, L. casei, L. fermentum, and B. bifidum) was used in two high-quality human RCTs, but lacked supporting mechanistic and/or animal model studies (BH score = 5; Table 5). Additional promising probiotic treatments with high BH scores included B. animalis, L. paracasei, and E. coli Nissle 1917, each receiving a BH score of 7; and L. plantarum, Lacto-mix, L. crispatus & L. rhamnosus, and the B. animalis combination therapy, all of which received a BH score of 6.
The majority of the remaining microbial treatments were characterized by low BH scores, resulting from a paucity of studies, lack of mechanistic evidence, and/or presence of conflicting evidence. The latter is exemplified by L. casei (BH score = 1), which was examined in six animal studies, but showed evidence of disease exacerbation or lack of effect in four of those studies, resulting in a deduction of 2 points for BHC #4. We note that this interpretation is confounded by the fact that different strains/isolates were used across these different studies, highlighting the need for careful standardization and interpretation.
BHC deficiencies
Using the Bradford Hill criteria, several therapies had considerable evidence for strength of relationship, dose–response relationship, biological plausibility, and coherence. Future studies should focus on strengthening these areas further by investigating dosing effects, establishing more direct evidence of MGBA involvement with more probiotic organisms and combinations, addressing alternative explanations, and repeating interventions in various contexts. Additionally, more evidence is needed to fulfill the remaining Bradford Hill criteria categories (BHC #s 1, 4, 6, and 7), including more before-and-after analyses, using a standardized protocol to facilitate comparisons across studies and research groups, and investigating cessation effects (Table 6). Strengthening the specificity of association by comparing the effects of live versus heat-killed organisms and their soluble products, and the inclusion of more mechanistic experiments is also recommended. Future studies and reviews should also consider the taxonomic reclassification of Lactobacillus spp. when referring to those probiotic organisms.77
Table 6.
Bradford Hill criteria (BHC) warranting most attention for future probiotic and commensal therapy studies
| Probiotic Therapy | Commensal Therapy | |
|---|---|---|
| BHC # | 1. Temporal relationship | 2. Strength of relationship |
| 4. Replication of findings | 3. Dose–response relationship | |
| 6. Cessation of exposure | 4. Replication of findings | |
| 7. Specificity of association | 6. Cessation of exposure | |
| 8. Coherence between multiple approaches |
Abbreviation: BHC, Bradford Hill criteria.
Lastly, to move toward translational application, wherein probiotics are stringently defined as conferring a known benefit to human health, disease-specific usage should be assessed in well-powered RCTs to provide clinically relevant guidance. Notably, a defined benefit to MS patient health should not be limited to clinical outcome, but also include secondary parameters such as quality of life, since it is plausible that probiotic therapy may improve the well-known GI-associated MS symptomatology (e.g. constipation) rather than affecting overall disease progression directly; and mental health, since depression has been identified as a risk factor for RRMS disability and relapses.78,79,80
C. Commensal therapy
Major trends
The major findings for each of the commensal therapy animal studies included in this review can be found in Table 4. A qualitative summary of these studies is provided below, followed by a semi-quantiative ranked evaluation using BH criteria.
Table 4.
Animal studies of commensal therapies: summary of study characteristics and major findings
| Study | ROB | Sample | MS Model | Intervention | Timeline | Duration | Major Findings |
|---|---|---|---|---|---|---|---|
| Montgomery et al.37 | Low | M & F C57BL6 & PWD mice 4 wko |
2× MOG-induced EAE |
T: L. reuteri isolated from PWD cecal contents (100 µL of 109 CFU o.g.) w/ 100 µL cryopreserved C57BL6 cecal microbiota C: 200 µL cryopreserved C57BL6 cecal microbiota |
Proph | 4 wks (one initial admin) |
CD: ↑ CDS & freq. of infiltrating CD4 + T cells in spinal cord; – freq. of infiltrating CD8 + T cells in spinal cord IM: ↑ freq. of GM-CSF- and IFN-γ- producing CD4+ & CD8 + T cells MM: NA MC: NA |
| Chen et al.65 | UN | F C57BL6 mice 3–4 wko n = 8/g |
2× MOG-induced EAE |
T1: C. butyricum GDBIO1501, 5 × 106 CFU/mL o.g. T2: norfloxacin, 5 mg/kg o.g. C1: PBS w/ EAE C2: normal control |
Proph | Daily for 3 wks pre-EAE |
CD: ↓ daily CS, lymphocyte infiltration, demyelinating plaques in lumbar spinal cord IM: ↓ TH17 cells in CNS, LN, colon, spleen, & small intestine, IFN-γ-producing CD4 + T cells in spleen; – IL-17A+IFN-γ+ CD4 + T cells; ↑ differentiation of Tregs MM: ↑ # of OTUs, abundance, diversity, and rel. abund. of Prevotella, Bacteriodetes; ↓ rel. abund. of Firmicutes, Desulfovibroneceae, Ruminococcus MC: ↓ phosphorylation of p38 MAPK, ERK1/2, and JNK in lumbar spinal cord |
| Mangalam et al.56 | UN | M & F HLA-DR3.DQ8 transgenic mice 8–12 wko n = 5–11/g |
PLP-induced EAE |
T1: Proph P. histicola, 108 CFU/mL o.g. T2: Proph P. melaninogenica, 108 CFU/mL o.g. T3: Proph C. sputigena, 108 CFU/mL o.g. All isolated from duodenum of celiac disease patients T4: Proph mouse-specific E. coli, 108 CFU/mL o.g. T5: T1 w/ Abx-depleted flora T6: Thera T1 T7: HK T6 T8: T6 cell-free supernatant T9: T6 107 CFU T10: T6 109 CFU C: medium |
Proph & Thera | Every other day for 2 wks (starting 7 d pre-EAE or 7 dpi) Abx depletion for 3 wks AT at 5 dpi |
CD: ↓ EAE incidence, CDS, regions of brain & spinal cord inflammation and demyelination, BBB permeability, CNS cellular infiltration (T1); earlier onset (T1); T1 restored gut permeability IM: ↓ IL-23, IL-12, IFN-γ, IL-17, CD4 + T cells, IFN-γ- and IL-17-expressing CD4 + T cells (T1); ↑ IL-10, TGF-β, CD4+ CD25+ Foxp3+ Tregs (T1); ↑ IL-10 (T2) MM: ↑ rel. abund. of Prevotella, Lactobacillus, Sutterella (T1), resembling pre-EAE states MC: Milder EAE (T5); ↑ EAE incidence (T7/T8); ↑ EAE suppression (T6 vs T9/T10); ↓ EAE incidence in AT recipient mice of T1 |
| Shahi et al.57 | UN | M & F HLA-DR3.DQ8 double transgenic mice & C57BL6 mice 8–12 wko n ≥ 7/g |
PLP-induced EAE (HLA) and MOG-induced EAE (C57BL6) |
T1: Proph P. histicola, 108 CFU o.g. in HLA mice T2: Proph Copaxone, 2 mg s.c. in HLA mice T3: T1+ T2 T4: T1 in C57BL6 mice T5: T2 in C57BL6 mice T6: T3 in C57BL6 mice T7: Thera T1 T8: Thera T2 T9: Thera T3 C: PBS or TSB media |
Proph & Thera | Every other day for 2 wks (starting 7 d pre-EAE or 7 dpi) T3, T6, T9 = T1 & T2 on alternating days |
CD: ↓ average daily scores & CDS (T2,T3,T4-T6,T7-T9); delayed EAE onset (T9); ↓ inflammation, demyelination (T1-T3); effects of T3/T6/T9 not more pronounced IM: ↓ IL-17+ CD4+ & IFN-γ+ CD4 + T cells in brain & spinal cord (T1,T3); ↑ CD4+ Foxp3+ Tregs in splenocytes and GALT (T1,T3); – IL-10-producing CD4 + T cells (T1-T3) MM: ↑ rel. abund. Lactobacillus (T1,C), ↓ (T2,T3) MC: NA |
| Shahi et al.58 | UN | M & F HLA-DR3.DQ8 double transgenic mice 8–12 wko n ≥ 12/g |
PLP-induced EAE |
T1: Proph P. histicola, 108 CFU o.g T2: Proph IFNβ, 10,000 IU T3: Proph T1+ T2 T4: Thera T1 T5: Thera T2 T6: Thera T3 C: TSB media |
Proph & Thera | Every other day for 2 wks pre-EAE (7 doses) or starting 7 dpi (7 doses) |
CD: ↓ average daily score & CDS (T4-T6), inflammatory cellular infiltration into brain & spinal cord (T4,T6), spinal cord tissue and meningeal/stratum regions of brain (T4-T6); no additive effects for T6 vs T4/T5 IM: ↓ Iba-1+ microglia & GFAP+ astrocytes in brain & spinal cord white matter, CD4+ IL-17+ & CD4+ IFN-γ + T cells (T1-T3); ↑ CD4+ Foxp3+ Tregs, IL-10-producing CD4 + T cells (T1,T3) MM: NA MC: NA |
| Takata et al.70 | UN | F C57BL6 mice & SJL mice 6 wko n = 17/g |
MOG-induced EAE (C57BL6) and PLP-induced EAE (SJL) |
T1: HK P. acidilactici R037 in C57BL6 mice, 20 mg/mL o.g. T2: HK P. acidilactici R037 in water in SJL mice, 0.8 mg/mL orally C1: PBS, o.g. C2: PBS in water, orally |
Proph & Thera | Daily for 36 d (starting 2 wks pre-EAE) |
CD: ↓ CS (T1,T2); ↓ infiltrating MNCs (T1); delayed EAE onset (T2) IM: ↓ IL-17 & IFN-γ in splenocytes & draining LNs; ↑ IL-10 & CD4+ IL-10 + T cells in mesenteric LNs and splenocytes MM: NA MC: NA |
| Miyauchi et al.38 | Low | F GF C57BL6 mice 5–7 wko n = 5–10/g |
MOG-induced EAE |
T1: OTU0001 (L. reuteri, 100% 16S rRNA match to strains H4 & LMG 18238) T2: OTU0002 (Allobaculum) Both isolated from small intestine of specific PF mice and o.g. at 5–7 wko for stable colonization. T3: T1 + T2 co-colonized T4: T3 with urvA-deficient L. reuteri C: naïve GF |
Proph | One admin at 5–7 wko |
CD: ↑ CS (T2,T3); – CS (T1); ↓ CS, EAE incidence (T4); ↑ demyelination, spinal cord infiltration, EAE incidence (T3) IM: ↑ IL-17A, TH17 cells in lamina propria of small intestine & splenocytes (T2); ↑ Tregs in small intestine (T2); ↑ TH17 cells (T3); – IL-17A, TH17 cells, Tregs in small intestine (T2/T3); – TH17 cells in small intestine (T1/T4) MM: – rel. abund. in small intestine (T2/T3); – rel. abund. in small intestine (T1/T4) MC: ↑ expression of Saa1, Saa2, Il23a, Il12b, Csf2, Il23r in small intestine (T2); – expression of Saa1, Saa2, Il23a in small intestine (T2/T3) |
| Ochoa-Reparaz et al.62 | UN | F SJL mice 6 wko n = 6–8/g |
PLP-induced EAE |
T1: 1 wk Abx, recolonize w/ WT B. fragilis NCTC 9343 T2: 1 wk Abx, recolonize w/ PSA-deficient B. fragilis Both at 1010 CFU in 200 µL sterile PBS o.g. C1: 1 wk Abx only C2: sham (no Abx, receive PBS) |
Proph | 30 d (one initial admin) |
CD: ↓ CS, CDS (T1,C1); delayed EAE onset (T1,C1); – EAE incidence IM: ↓ Tbet (C1), IFN-γ (C1), RORγt (T1,C1), IL-17 (T1,C1); ↑ GATA-3 (T1,C1), IL-10 (T1,C1), SMAD-3 (T1), IL-13 (C1), freq. Foxp3+ CD25+ CD4 + T cells in cervical LNs (C1); ↑ IL-17, RORγt, Tbet and ↓ GATA-3, IL-10, IL-13 (T2); ↓ conversion of CD103+ DCs to Foxp3+ Tregs (T2) MM: # of detectable bacteria after Abx restored (T1,T2) (colonization confirmed) MC: Depletion of CD25 + T cells eliminated protective effects (C1,T1); ↓ CS & ↑ IL-10 in AT recipient mice (T1 only) |
| Liu et al.35 | UN | F C57BL6 mice 6–8 wko n = 23–28/g |
MOG-induced EAE |
T1: Akkermansia muciniphila ATCC BAA-835 T2: E. coli K-12 Strain #7296 C: medium |
Thera | Daily for 7 d (starting 11 dpi) |
CD: ↓ CS, demyelination, axonal loss (T1) IM: ↑ MOG-specific Foxp3+ Tregs and total Tregs in spleen (T1); ↑ Foxp3+ Tregs from DCs (T1); – direct Foxp3+ Treg induction (T1 vs T2); ↓ IL-6, IL-1b expression in DCs (T1), – TGF-β expression (T1/T2) MM: NA MC: NA |
All findings are reported with respect to control group(s) unless otherwise indicated.
Key: ↓ decreased; ↑ increased; – no change or no difference compared to control; NA, not applicable to this study.
Abbreviations: ROB, risk of bias; MS, multiple sclerosis; UN, uncertain; M, male; F, female; wk/wks/wko, week/weeks/weeks old; g, group; EAE, experimental autoimmune encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein; PLP, proteolipid protein; T, treatment group; C, control; CFU, colony-forming units; o.g., oral gavage; PBS, phosphate-buffered saline; s.c., subcutaneous; Abx, antibiotics; HK, heat-killed; IFNβ, interferon beta; IU, international unit; OTU, operational taxonomic unit; PF, pathogen-free; GF, germ-free; WT, wild-type; PSA, polysaccharide A; Thera, therapeutic; Proph, prophylactic; admin; administration; dpi, days post immunization; CD, clinical disease; IM, immune/metabolic; MM, microbiome/metabolome; MC, mechanistic/correlative; CDS, cumulative disease score; freq., frequency; GM-CSF, granulocyte monocyte colony-stimulating factor; IFN-γ, interferon gamma; CS, clinical score; CNS, central nervous system; LN, lymph node; IL, interleukin; rel. abund., relative abundance; BBB, blood–brain barrier; AT, adoptive transfer; GALT, gut-associated lymphoid tissue; DC, dendritic cell; TGF-β, transforming growth factor beta; MNC, mononuclear cell.
No human studies were identified for commensal therapy in this review, so the below trends are limited to preclinical findings. All but two (L. reuteri37,38 and Allobaculum38) of the commensal organisms studied among the nine studies were shown to delay EAE onset and decrease clinical scores, incidence, inflammatory CNS infiltration, and demyelination. P. histicola was one of two commensals represented in more than one study and exhibited positive outcomes in each.56–58 One study also reported reduced astrocytosis and microglial activation in the brain and spinal cord of P. histicola-treated mice,58 while a sister study found that P. histicola helped to strengthen the MGBA by decreasing BBB permeability and restoring gut permeability.56 Similar to probiotic therapy, reduced proinflammatory and increased anti-inflammatory immune responses were observed in each of the commensal studies. Specifically, studies found decreased IL-17- and IFN-γ-producing CD4 + T cells,56,58,65 TH17 cells,65 and IL-17,56,62,70 IFN-γ,56,70 IL-23,56 and IL-1270 cytokines; and increased IL-10,56,62,70 TGF-β,56 and Tregs.56–58,65 These results were consistent across all three P. histicola treatments.56,57,58 L. reuteri was also represented in multiple studies and was shown to exacerbate EAE in both, either when administered alone (in the context of a normal microbiome)37 or in combination with Allobaculum (in a dual-colonization gnotobiotic model).38
For studies that analyzed the microbiome, commensal therapy groups had a general microbiome shift toward pre-EAE states following treatment, including an increased relative abundance of Bacteriodetes, Firmicutes, Prevotella spp., and Lactobacillus spp.56,57,65 Mechanistically, the adoptive transfer of splenocytes from P. histicola-treated mice led to decreased EAE incidence in recipient mice.56 Similarly, the adoptive transfer of FoxP3+ cells from wild-type B. fragilis-treated mice, resulted in decreased EAE clinical scores and increased levels of IL-10 in recipient mice.62 These findings were not observed in the recipient mice receiving cells from polysaccharide A (PSA)-deficient B. fragilis-treated mice, suggesting that PSA is requisite for EAE protection. Separately, treatment with C. butyricum was reported to suppress phosphorylation of p38 MAPK and JNK signaling pathways – which are typically elevated in EAE – in the spinal cords of mice.65 One commensal was also found to be at least as effective as two different DMTs (glatiramer acetate57 and IFNβ58).
BHC scores and rankings
The BH score calculations and findings for commensal therapy are detailed in Supplemental File 2, and summarized in Table 5.
Treatment with P. histicola had fairly strong evidence (BH score = 7), but fell short across several BH categories (BHC #s 4 and 6), as it lacked human studies and replication by independent groups (Table 5). Another promising treatment with a high BH score was B. fragilis (BH score = 5), which scored points in BHC # 1, 2, 5, 7, and 8 owing to an adoptive transfer experiment, but lacked replication and evidence of dose-response and cessation effects. The remaining treatments were characterized by low BH scores (ranging 1–4) comprised of points in BHC # 1, 2, 3, and/or 5, once again resulting from a paucity of studies, lack of mechanistic evidence, and/or presence of conflicting evidence, as observed with L. reuteri (BH score = 1), which was found to exacerbate the disease in three of the five studies, leading to a 2-point deduction for BHC #4. As noted with L. casei for probiotic therapy, this interpretation is confounded by the use of different strains/isolates and modes of treatment (stable commensal colonization vs. daily gavage) across studies and would benefit from careful standardization (Table 5).
BHC deficiencies
Using the Bradford Hill criteria, commensal therapy had strong evidence for temporal relationship, specificity of association, and biological plausibility, but was lacking in the remaining categories (BHC #s 2–4, 6, and 8; Table 6). Additional studies replicating current findings and testing more commensal organisms and combinations should be the main focus of future studies, since there were few commensal therapy studies overall and only P. histicola and L. reuteri were used in more than one study. Future studies should also focus on confirming colonization of the commensal organisms in the gut to reduce confounding and strengthen the specificity of association (BHC #7). Testing the effects of live versus heat-killed organisms and their products should also be prioritized, as these findings may contribute to the elucidating the underlying therapeutic mechanisms. For instance, subsequent studies of B. fragilis by the same research group utilized only the B. fragilis PSA symbiosis factor rather than administering live, wild-type B. fragilis and found similar reductions in EAE severity, as well as protection against EAE demyelination and inflammatory responses, providing a key molecular mechanism in support of the action of the live bacterium.81–84
Other recommendations reflect those of probiotic therapy, namely controlling for alternative explanations, supporting immunological and microbiological findings with mechanistic experiments, and adding standardization to promote study design consistency and ease of comparison across studies.
Discussion
The purpose of this comprehensive review was to compile, summarize, and systematically rank the current evidence for probiotic and commensal therapeutic efficacy in MS and its preclinical models in an effort to identify weaker areas that should be addressed in future studies. A total of 37 studies were evaluated, including 28 for probiotic therapy and 9 for commensal therapy. The probiotic formulations VSL#3 (BH score = 9), B. animalis, L. paracasei, and E. coli Nissle 1917 (BH scores = 7) ranked highest due to their fulfillment of at least six of the eight Bradford Hill criteria. For commensal therapy – which suffered from a complete absence of clinical studies – the highest rankings went to P. histicola (BH score = 7) and B. fragilis (BH score = 5).
Animal studies demonstrated generally higher efficacy for reducing disease severity and progression with probiotic therapy than did the human studies, which is not unexpected, given the known shortcomings of the animal models, and the expected difficulties in translating basic science findings into therapy. The disconnect between human and animal studies could also be due to the difference and extent of the clinical markers measured, as clinical studies only measured MS severity through EDSS and questionnaires, while the pre-clinical studies were able to investigate EAE and the other MS models more comprehensively. MRI evaluation is a powerful, unbiased, and quantitative surrogate for MS severity and progression, and this was conspicuously lacking in the human studies. Additionally, human studies were in all likelihood underpowered to detect potentially subtle effects of probiotic treatments, and confounded by multiple environmental variables (e.g. diet, host baseline differences) that are impossible to control in this setting.
Replication of findings (BHC #4) was one of the most deficient Bradford Hill criteria across studies, with only 25% (n = 28) of the formulations receiving points and two of them (L. reuteri and L. casei) losing points. Another almost uniformly unfulfilled criterion was cessation of exposure, only addressed by six formulations. Both probiotic and commensal therapies would benefit from additional replication, testing more organism combinations, improved mechanistic evidence and comparison of live versus heat-killed organisms and their soluble products, and protocol standardization to enable improved contextual comparison across studies.
Limitations
Study limitations
There were several limitations to accurately assessing the efficacies of both therapies. First, there was widespread study design variability in the species and/or strain, dosage, duration of intervention, timeline, and sample characteristics. These variations would have been beneficial to external validation if the same strains were used across studies, but instead posed a challenge for assessing therapeutic utility. Standardized protocols outlining the optimal dosage, timeline, and duration for different organisms would be helpful for mitigating this issue, as was the focus of a review on probiotic therapy that concluded 109 CFU for 8–12 weeks duration produced the most favorable results.31
Another study design issue was exclusion criteria and control of confounding variables, since some of the human studies did not account for diet or stress, which can alter gut microbial composition and subsequently influence MGBA interactions and concurrent DMT use, which could overshadow the true therapeutic efficacy if synergism exists between the two.34 Additionally, genetic variability was also mostly unaccounted for in both human and animal studies (since the latter for the most part used a single strain of mouse). Furthermore, none of the human studies were conducted long enough to span the average remission period of 12–18 months, so the true impact of each therapy on reducing the severity of MS cannot be revealed with certainty.1,85 As for animal studies, none of these can accurately capture the complexity of spontaneous MS and its various forms in humans.86–88
Only five studies tested the effects of live versus heat-killed organisms or their products.48,49,53,56,62 This distinction is important, since equivalent efficacy with heat-killed organisms would help to reduce any associated risks of therapy posed by live microbiota and likely improve treatment uptake and adherence in patients. Furthermore, this effect likely differs across organisms. For instance, L. paracasei,49 a combination of L. crispatus and L. rhamnosus,53 and a combination of two B. animalis subsp. lactis strains53 did not need to be viable for EAE suppression, while P. histicola56 and Lacto-mix48 did. Additionally, protection from EAE elicited by B. fragilis required the expression PSA by this bacterium, indicating that this bacterial product alone can play an important role in EAE protection.62 Indeed, follow-up studies confirmed that live B. fragilis is not required, while PSA is sufficient to elicit a therapeutic effects.81–84 Future studies should prioritize these distinctions to help optimize efficacy and therapeutic success.
Another limitation of this review was the quality and risk of bias for the studies included. Most of the animal studies included were classified as “medium quality” with “uncertain” bias due to the lack of explicitly stated randomization and blinding measures used. A lack of randomization can subject the results to inadvertent confounding and chance findings.39,40 Animals that live together in the same cage or area of a room may have more similar characteristics to each other than compared to a different cage or area importantly including the basal composition of their gut microbiomes. Additionally, a lack of blinding can introduce both performance and detection bias, as a researcher or caretaker’s knowledge of the treatment group can cause them to subconsciously act differently toward one group, such as providing extra care to sicker animals.39,40 It is entirely possible that these studies did in fact incorporate these measures into their protocol, but since it was not stated it was considered to be absent for this review. Other issues regarding quality include the premise that the positive findings observed across studies may simply reflect consistency of confounding variables and/or publication bias, rather than the therapy itself.
Review limitations
As for the risk of bias for this review, there were several methodological flaws in the assessment of therapeutic efficacy. Personal judgment was required for assessing each therapy’s fulfillment of the Bradford Hill criteria and there were no pre-defined guidelines as to what should be considered “sufficient evidence.” The scoring system was implemented as an arbitrary method to facilitate comparison of the efficacies in the context of the recommendation criterion and not intended to be a comprehensive assessment of the therapies. Regardless, the aim of this review was to highlight areas within each therapy that should be strengthened in future studies, so the risk of bias in this sense seems low. Separately, a few of the Bradford Hill criteria may be less important for establishing efficacy, causing the therapeutic to be penalized for lacking evidence in a non-applicable category. For example, a threshold effect rather than a dose–response relationship might be necessary for observing beneficial effects. Cessation of exposure may also not be necessary to demonstrate, since these therapies would theoretically be lifelong as is the case for DMTs. Accordingly, another avenue for future research could be establishing a minimum set of probiotic specific criteria for comprehensively evaluating therapeutic strategies in both animal and human studies.
Other limitations of this review were related to the search and screening process for identifying relevant studies. First, the use of an English language filter in our search strategy imposed obvious restrictions on the number of studies included and extent of available evidence. Second, the operational definitions we established limited the scope of probiotic and commensal therapies to only live or heat-killed bacteria, excluding any mechanistic evidence that may have been generated in studies that used only probiotic/commensal strain-soluble products. The BH score for B fragilis, for example, could have been improved had the follow-up studies that focused on the B. fragilis PSA symbiosis factor been eligible for inclusion.81–84 Lastly, we did not contact the authors of studies classified as medium or low quality for clarification of missing methodological data (i.e. randomization, blinding). Attaining such information could have altered our quality assessments and evaluations for BHC #2. Regardless of these limitations, this review was intended as a resource to guide and optimize future probiotic and commensal therapy studies by highlighting both emerging therapies and study shortcomings, rather than to firmly conclude the therapeutic utility of specific formulations.
Conclusion
In this comprehensive review, we used a Bradford Hill criteria scoring approach to provide a multi-parameter assessment and ranking of evidence for specific gut microbial therapies, with the overall goal of identifying and highlighting areas of need for future research (see Tables 5 and 6). Several formulations emerged as having the most promise, including VSL#3, B. animalis, L. paracasei, and E. coli Nissle 1917 for probiotics; and P. histicola and B. fragilis for commensals. However, many other therapies fell short across a number of criteria, notably replication of findings. Other Bradford Hill criteria lacking evidence were temporal relationship and specificity of association for probiotic therapy, and strength of relationship, dose–response relationship, and coherence for commensal therapy. Future studies should prioritize addressing these shortcomings through better control of confounding, supporting immunological and microbiological findings with mechanistic experiments, improved standardization of protocols and therapeutic formutions, and the other suggestions discussed in this review. Focusing on these areas is necessary to make progress toward clinical implementation, since cheaper, safer, and more durable treatments for MS are in demand.
Supplementary Material
Funding Statement
This work was supported by NIH/NINDS to DNK [R01 NS097596]. TM was supported by Vermont Center for Immunology / Infectious Diseases training grant, 5 T32 AI055402 from the National Institute of Allergy and Infectious Diseases.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Baecher-Allan C, Kaskow BJ, Weiner HL.. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97(4):742–27. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Robertson D, Moreo N. Disease-modifying therapies in multiple sclerosis: overview and treatment considerations. Fed Pract. 2016;33:28–34. [PMC free article] [PubMed] [Google Scholar]
- 3.Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816–1821. doi: 10.1177/1352458520970841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Förster M, Küry P, Aktas O, Warnke C, Havla J, Hohlfeld R, Mares J, Hartung H-P, Kremer D. Managing risks with immune therapies in multiple sclerosis. Drug Saf. 2019;42(5):633–647. doi: 10.1007/s40264-018-0782-8 [DOI] [PubMed] [Google Scholar]
- 5.Harding K, Williams O, Willis M, Hrastelj J, Rimmer A, Joseph F, Tomassini V, Wardle M, Pickersgill T, Robertson N, Tallantyre E. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76(5):536. doi: 10.1001/jamaneurol.2018.4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yano H, Gonzalez C, Healy BC, Glanz BI, Weiner HL, Chitnis T. Discontinuation of disease-modifying therapy for patients with relapsing-remitting multiple sclerosis: effect on clinical and MRI outcomes. Mult Scler Relat Disord. 2019;35:119–127. doi: 10.1016/j.msard.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Baroncini D, Zaffaroni M, Moiola L, Lorefice L, Fenu G, Iaffaldano P, Simone M, Fanelli F, Patti F, D’Amico E, et al. Long-term follow-up of pediatric MS patients starting treatment with injectable first-line agents: a multicentre, Italian, retrospective, observational study. Mult Scler. 2019;25(3):399–407. doi: 10.1177/1352458518754364 [DOI] [PubMed] [Google Scholar]
- 8.Gajofatto A, Bacchetti P, Grimes B, High A, Waubant E. Switching first-line disease-modifying therapy after failure: impact on the course of relapsing–remitting multiple sclerosis. Mult Scler. 2009;15(1):50–58. doi: 10.1177/1352458508096687. [DOI] [PubMed] [Google Scholar]
- 9.Hua LH, Harris H, Conway D, Thompson NR. Changes in patient-reported outcomes between continuers and discontinuers of disease modifying therapy in patients with multiple sclerosis over age 60. Mult Scler Relat Disord. 2019;30:252–256. doi: 10.1016/j.msard.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004;3(12):709–718. doi: 10.1016/S1474-4422(04)00933-0. [DOI] [PubMed] [Google Scholar]
- 11.Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010;9(7):727–739. doi: 10.1016/S1474-4422(10)70094-6. [DOI] [PubMed] [Google Scholar]
- 12.Boziki MK, Kesidou E, Theotokis P, Mentis A-FA, Karafoulidou E, Melnikov M, Sviridova A, Rogovski V, Boyko A, Grigoriadis N. Microbiome in multiple sclerosis: where are we, what we know and do not know. Brain Sci. 2020;10(4):234. doi: 10.3390/brainsci10040234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galland L. The gut microbiome and the brain. J Med Food. 2014;17(12):1261–1272. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camara-Lemarroy CR, Metz LM, Yong VW. Focus on the gut-brain axis: multiple sclerosis, the intestinal barrier and the microbiome. WJG. 2018;24(37):4217–4223. doi: 10.3748/wjg.v24.i37.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183(10):6041–6050. doi: 10.4049/jimmunol.0900747 [DOI] [PubMed] [Google Scholar]
- 16.Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7(1):12015. doi: 10.1038/ncomms12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain. 2018;141(7):1900–1916. doi: 10.1093/brain/awy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim S-W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. Wilson BA, ed. ONE PLoS. 2015;10(9):e0137429. doi: 10.1371/journal.pone.0137429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci USA. 2017;114(40):10719–10724. doi: 10.1073/pnas.1711233114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, Luckey DH, Marietta EV, Jeraldo PR, Chen X, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6(1):28484. doi: 10.1038/srep28484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang C-J, Lin T-L, Tsai Y-L, Wu T-R, Lai W-F, Lu C-C, Lai H-C. Next generation probiotics in disease amelioration. J Food Drug Anal. 2019;27(3):615–622. doi: 10.1016/j.jfda.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Liu M, Cao J, Li X, Fan D, Xia Y, Lu X, Li J, Ju D, Zhao H. The dynamic interplay between the gut microbiota and autoimmune diseases. J Immunol Res. 2019;2019:1–14. doi: 10.1155/2019/7546047 [DOI] [PMC free article] [PubMed]
- 23.Smallwood TB, Giacomin PR, Loukas A, Mulvenna JP, Clark RJ, Miles JJ. Helminth immunomodulation in autoimmune disease. Front Immunol. 2017;8:453. doi: 10.3389/fimmu.2017.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correale J. Helminth/parasite treatment of multiple sclerosis. Curr Treat Options Neurol. 2014;16(6):296. doi: 10.1007/s11940-014-0296-3. [DOI] [PubMed] [Google Scholar]
- 25.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 26.Dargahi N, Johnson J, Donkor O, Vasiljevic T, Apostolopoulos V. Immunomodulatory effects of probiotics: can they be used to treat allergies and autoimmune diseases? Maturitas. 2019;119:25–38. doi: 10.1016/j.maturitas.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Bach J-F. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol. 2018;18(2):105–120. doi: 10.1038/nri.2017.111. [DOI] [PubMed] [Google Scholar]
- 28.Calvo-Barreiro L, Eixarch H, Montalban X, Espejo C. Combined therapies to treat complex diseases: the role of the gut microbiota in multiple sclerosis. Autoimmun Rev. 2018;17(2):165–174. doi: 10.1016/j.autrev.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Guarner F, Bourdet-Sicard R, Brandtzaeg P, Gill HS, McGuirk P, van Eden W, Versalovic J, Weinstock JV, Rook GA. Mechanisms of Disease: the hygiene hypothesis revisited. Nat Rev Gastroenterol Hepatol. 2006;3(5):275–284. doi: 10.1038/ncpgasthep0471 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Alookaran J, Rhoads J. Probiotics in autoimmune and inflammatory disorders. Nutrients. 2018;10(10):1537. doi: 10.3390/nu10101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morshedi M, Hashemi R, Moazzen S, Sahebkar A, Hosseinifard E-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: a systematic review. J Neuroinflammation. 2019;16(1):231. doi: 10.1186/s12974-019-1611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoledziewska M. The gut microbiota perspective for interventions in MS. Autoimmun Rev. 2019;18(8):814–824. doi: 10.1016/j.autrev.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Jiang J, Chu C, Wu C, Wang C, Zhang C, Li T, Zhai Q, Yu L, Tian F, Chen W. Efficacy of probiotics in multiple sclerosis: a systematic review of preclinical trials and meta-analysis of randomized controlled trials. Food Funct. 2021;12(6):2354–2377. doi: 10.1039/D0FO03203D [DOI] [PubMed] [Google Scholar]
- 34.Kohl HM, Castillo AR, Ochoa-Repáraz J. The microbiome as a therapeutic target for multiple sclerosis: can genetically engineered probiotics treat the disease? Diseases. 2020;8(3):33. doi: 10.3390/diseases8030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Rezende RM, Moreira TG, Tankou SK, Cox LM, Wu M, Song A, Dhang FH, Wei Z, Costamagna G, Weiner HL. Oral administration of mir-30d from feces of MS patients suppresses MS-like symptoms in mice by expanding Akkermansia muciniphila. Cell Host Microbe. 2019;26(6):779–794.e8. doi: 10.1016/j.chom.2019.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guarner F, Sanders ME, Eliakim R, Fedorak R, Gangl A, Garisch J, Kaufmann P, Karakan T, Khan AG, Kim N, et al. World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics. World Gastroenterology Organisation. 2017. [accessed 2021. Feb 1] https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf
- 37.Montgomery TL, Künstner A, Kennedy JJ, Fang Q, Asarian L, Culp-Hill R, D’Alessandro A, Teuscher C, Busch H, Krementsov DN. Interactions between host genetics and gut microbiota determine susceptibility to CNS autoimmunity. Proc Natl Acad Sci USA. 2020;117(44):27516–27527. doi: 10.1073/pnas.2002817117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyauchi E, Kim S-W, Suda W, Kawasumi M, Onawa S, Taguchi-Atarashi N, Morita H, Taylor TD, Hattori M, Ohno H. Gut microorganisms act together to exacerbate inflammation in spinal cords. Nature. 2020;585(7823):102–106. doi: 10.1038/s41586-020-2634-9 [DOI] [PubMed] [Google Scholar]
- 39.Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct182):d5928–d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed]
- 40.Hooijmans CR, Rovers MM, De Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1):43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epidemiology. Philadelphia (PA):: Elsevier/Saunders;; 2014. [Google Scholar]
- 43.Mestre L, Carrillo-Salinas FJ, Feliú A, Mecha M, Alonso G, Espejo C, Calvo-Barreiro L, Luque-García JL, Estevez H, Villar LM, Guaza C. How oral probiotics affect the severity of an experimental model of progressive multiple sclerosis? Bringing commensal bacteria into the neurodegenerative process. Gut Microbes. 2020;12(1):1813532. doi: 10.1080/19490976.2020.1813532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calvo-Barreiro L, Eixarch H, Ponce-Alonso M, Castillo M, Lebrón-Galán R, Mestre L, Guaza C, Clemente D, del Campo R, Montalban X, Espejo C. A commercial probiotic induces tolerogenic and reduces pathogenic responses in experimental autoimmune encephalomyelitis. Cells. 2020;9(4):906. doi: 10.3390/cells9040906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMurran CE, Guzman de la Fuente A, Penalva R, Ben Menachem-Zidon O, Dombrowski Y, Falconer J, Gonzalez GA, Zhao C, Krause FN, Young AMH, et al. The microbiota regulates murine inflammatory responses to toxin-induced CNS demyelination but has minimal impact on remyelination. Proc Natl Acad Sci USA. 2019;116(50):25311–25321. doi: 10.1073/pnas.1905787116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, Kivisäkk P, Pierre IV, Hrishikesh L, Gandhi R, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. 2018;83(6):1147–1161. doi: 10.1002/ana.25244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tankou SK, Regev K, Healy BC, Cox LM, Tjon E, Kivisakk P, Vanande IP, Cook S, Gandhi R, Glanz B, et al. Investigation of probiotics in multiple sclerosis. Mult Scler. 2018;24(1):58–63. doi: 10.1177/1352458517737390 [DOI] [PubMed] [Google Scholar]
- 48.Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, Thorlacius H, Alenfall J, Jeppsson B, Weström B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. Unutmaz D, ed. ONE PLoS. 2010;5(2):e9009. doi: 10.1371/journal.pone.0009009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez JMS, Doty DJ, DePaula-Silva AB, Brown DG, Bell R, Klag KA, Truong A, Libbey JE, Round JL, Fujinami RS. Molecular patterns from a human gut-derived Lactobacillus strain suppress pathogenic infiltration of leukocytes into the central nervous system. J Neuroinflammation. 2020;17(1):291. doi: 10.1186/s12974-020-01959-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salehipour Z, Haghmorad D, Sankian M, Rastin M, Nosratabadi R, Soltan Dallal MM, Tabasi N, Khazaee M, Nasiraii LR, Mahmoudi M. Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomedicine Pharmacother. 2017;95:1535–1548. doi: 10.1016/j.biopha.2017.08.117 [DOI] [PubMed]
- 51.Ezendam J, De Klerk A, Gremmer ER, Van Loveren H. Effects of Bifidobacterium animalis administered during lactation on allergic and autoimmune responses in rodents: immune effects of probiotics. Clin Exp Immunol. 2008;154(3):424–431. doi: 10.1111/j.1365-2249.2008.03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goudarzvand M, Rasouli Koohi S, Khodaii Z, Soleymanzadeh Moghadam S. Probiotics Lactobacillus plantarum and Bifidobacterium B94: cognitive function in demyelinated model. Med J Islam Repub Iran. 2016;30:391. [PMC free article] [PubMed] [Google Scholar]
- 53.Consonni A, Cordiglieri C, Rinaldi E, Marolda R, Ravanelli I, Guidesi E, Elli M, Mantegazza R, Baggi F. Administration of Bifidobacterium and Lactobacillus strains modulates experimental myasthenia gravis and experimental encephalomyelitis in Lewis rats. Oncotarget. 2018;9(32):22269–22287. doi: 10.18632/oncotarget.25170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Libbey JE, Sanchez JMS, Fleming BA, Doty DJ, DePaula-Silva AB, Mulvey MA, Fujinami RS. Modulation of experimental autoimmune encephalomyelitis through colonisation of the gut with Escherichia coli. Benef Microbes. 2020;11(7):669–684. doi: 10.3920/BM2020.0012 [DOI] [PubMed] [Google Scholar]
- 55.Secher T, Kassem S, Benamar M, Bernard I, Boury M, Barreau F, Oswald E, Saoudi A. Oral administration of the probiotic strain Escherichia coli Nissle 1917. reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front Immunol. 2017;8:1096. doi: 10.3389/fimmu.2017.01096 [DOI] [PMC free article] [PubMed]
- 56.Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, Choung RS, Ju J, Sompallae R, Gibson-Corley K, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 2017;20(6):1269–1277. doi: 10.1016/j.celrep.2017.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shahi SK, Freedman SN, Murra AC, Zarei K, Sompallae R, Gibson-Corley KN, Karandikar NJ, Murray JA, Mangalam AK. Prevotella histicola, a human gut commensal, is as potent as Copaxone® in an animal model of multiple sclerosis. Front Immunol. 2019;10:462. doi: 10.3389/fimmu.2019.00462 [DOI] [PMC free article] [PubMed]
- 58.Shahi SK, Jensen SN, Murra AC, Tang N, Guo H, Gibson-Corley KN, Zhang J, Karandikar NJ, Murray JA, Mangalam AK. Human commensal Prevotella histicola ameliorates disease as effectively as interferon-beta in the experimental autoimmune encephalomyelitis. Front Immunol. 2020;11:578648. doi: 10.3389/fimmu.2020.578648 [DOI] [PMC free article] [PubMed]
- 59.Maassen CBM, JCAM van Holten, Balk F, den Bak‐Glashouwer MJH, Leer R, Laman JD, Boersma WJA, Claassen E. Orally administered Lactobacillus strains differentially affect the direction and efficacy of the immune response. Veterinary Quarterly. 1998;20(sup3):81–83. doi: 10.1080/01652176.1998.9694976 [DOI] [PubMed]
- 60.Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, Tajabadi-Ebrahimi M, Jafari P, Asemi Z. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutrition. 2017;36(5):1245–1249. doi: 10.1016/j.clnu.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 61.Tamtaji OR, Kouchaki E, Salami M, Aghadavod E, Akbari E, Tajabadi-Ebrahimi M, Asemi Z. The effects of probiotic supplementation on gene expression related to inflammation, insulin, and lipids in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. J Am Coll Nutr. 2017;36(8):660–665. doi: 10.1080/07315724.2017.1347074 [DOI] [PubMed] [Google Scholar]
- 62.Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide a expression. JI. 2010;185(7):4101–4108. doi: 10.4049/jimmunol.1001443 [DOI] [PubMed] [Google Scholar]
- 63.Rahimlou M, Hosseini SA, Majdinasab N, Haghighizadeh MH, Husain D. Effects of long-term administration of Multi-Strain Probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Nutr Neurosci. 2020;1–12. Published online June 5. doi: 10.1080/1028415X.2020.1758887. [DOI] [PubMed] [Google Scholar]
- 64.Salami M, Kouchaki E, Asemi Z, Tamtaji OR. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? A double blind clinical trial. J Funct Foods. 2019;52:8–13. doi: 10.1016/j.jff.2018.10.023. [DOI] [Google Scholar]
- 65.Chen H, Ma X, Liu Y, Ma L, Chen Z, Lin X, Si L, Ma Xueying, Chen X. Gut microbiota interventions with Clostridium butyricum and norfloxacin modulate immune response in experimental autoimmune encephalomyelitis mice. Front Immunol. 2019;10:1662. doi: 10.3389/fimmu.2019.01662 [DOI] [PMC free article] [PubMed]
- 66.Abdurasulova IN, Matsulevich AV, Tarasova EA, Kudryavtsev IV, Serebrjakova MK, Ermolenko EI, Bisaga GN, Klimenko VM, Suvorov AN. Enterococcus faecium strain L-3 and glatiramer acetate ameliorate experimental allergic encephalomyelitis in rats by affecting different populations of immune cells. Benef Microbes. 2016;7(5):719–729. doi: 10.3920/BM2016.0018 [DOI] [PubMed] [Google Scholar]
- 67.Yamashita M, Ukibe K, Matsubara Y, Hosoya T, Sakai F, Kon S, Arima Y, Murakami M, Nakagawa H, Miyazaki T. Lactobacillus helveticus SBT2171 attenuates experimental autoimmune encephalomyelitis in mice. Front Microbiol. 2018;8:2596. doi: 10.3389/fmicb.2017.02596 [DOI] [PMC free article] [PubMed]
- 68.Kobayashi T, Kato I, Nanno M, Shida K, Shibuya K, Matsuoka Y, Onoue M. Oral administration of probiotic bacteria, Lactobacillus casei and Bifidobacterium breve, does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis. Immunopharmacol Immunotoxicol. 2010;32(1):116–124. doi: 10.3109/08923970903200716 [DOI] [PubMed] [Google Scholar]
- 69.Kwon H-K, Kim G-C, Kim Y, Hwang W, Jash A, Sahoo A, Kim J-E, Nam JH, Im S-H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clinical Immunol. 2013;146(3):217–227. doi: 10.1016/j.clim.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 70.Takata K, Kinoshita M, Okuno T, Moriya M, Kohda T, Honorat JA, Sugimoto T, Kumanogoh A, Kayama H, Takeda K, et al. The lactic acid bacterium Pediococcus acidilactici suppresses autoimmune encephalomyelitis by inducing IL-10-producing regulatory T cells. Ashour HM, ed. ONE PLoS. 2011;6(11):e27644. doi: 10.1371/journal.pone.0027644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gharehkhani Digehsara S, Name N, Esfandiari B, Karim E, Taheri S, Tajabadi-Ebrahimi M, Arasteh J. Effects of Lactobacillus casei strain T2 (LBRC-M10783) on the modulation of Th17/Treg and evaluation of miR-155, miR-25, and IDO-1 expression in a cuprizone-induced C57BL/6 mouse model of demyelination. Inflammation. 2021;44(1):334–343. doi: 10.1007/s10753-020-01339-1 [DOI] [PubMed] [Google Scholar]
- 72.Ezendam J, Van Loveren H. Lactobacillus casei Shirota administered during lactation increases the duration of autoimmunity in rats and enhances lung inflammation in mice. Br J Nutr. 2008;99(1):83–90. doi: 10.1017/S0007114507803412. [DOI] [PubMed] [Google Scholar]
- 73.Baken KA, Ezendam J, Gremmer ER, de Klerk A, Pennings JLA, Matthee B, Peijnenburg AACM, van Loveren H. Evaluation of immunomodulation by Lactobacillus casei Shirota: immune function, autoimmunity and gene expression. Int J Food Microbiol. 2006;112(1):8–18. doi: 10.1016/j.ijfoodmicro.2006.06.009 [DOI] [PubMed] [Google Scholar]
- 74.Kobayashi T, Suzuki T, Kaji R, Serata M, Nagata T, Ando M, Iizuka R, Tsujibe S, Murakami J, Kiyoshima-Shibata J, et al. Probiotic upregulation of peripheral IL-17 responses does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis mouse models. Immunopharmacol Immunotoxicol. 2012;34(3):423–433. doi: 10.3109/08923973.2010.617755 [DOI] [PubMed] [Google Scholar]
- 75.He B, Hoang TK, Tian X, Taylor CM, Blanchard E, Luo M, Bhattacharjee MB, Freeborn J, Park S, Couturier J, et al. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front Immunol. 2019;10:385. doi: 10.3389/fimmu.2019.00385 [DOI] [PMC free article] [PubMed]
- 76.Johanson DM, Goertz JE, Marin IA, Costello J, Overall CC, Gaultier A. Experimental autoimmune encephalomyelitis is associated with changes of the microbiota composition in the gastrointestinal tract. Sci Rep. 2020;10(1):15183. doi: 10.1038/s41598-020-72197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, O’Toole PW, Pot B, Vandamme P, Walter J, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782–2858. doi: 10.1099/ijsem.0.004107 [DOI] [PubMed] [Google Scholar]
- 78.Bsteh G, Ehling R, Lutterotti A, Hegen H, Di Pauli F, Auer M, Deisenhammer F, Reindl M, Berger T. Long term clinical prognostic factors in relapsing-remitting multiple sclerosis: insights from a 10-year observational study. Meuth SG, ed. ONE PLoS. 2016;11(7):e0158978. doi: 10.1371/journal.pone.0158978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McIvor GP, Riklan M, Reznikoff M. Depression in multiple sclerosis as a function of length and severity of illness, age, remissions, and perceived social support. J Clin Psychol. 1984;40(4):1028–1033. doi:. [DOI] [PubMed] [Google Scholar]
- 80.Moore P, Hirsta C, Harding KE, Clarkson H, Pickersgilla TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272–276. doi: 10.1016/j.jpsychores.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3(5):487–495. doi: 10.1038/mi.2010.29 [DOI] [PubMed] [Google Scholar]
- 82.Telesford KM, Yan W, Ochoa-Reparaz J, Pant A, Kircher C, Christy MA, Begum-Haque S, Kasper DL, Kasper LH. A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39 + Foxp3 + T cells and T reg function. Gut Microbes. 2015;6(4):234–242. doi: 10.1080/19490976.2015.1056973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, Begum-Haque S, Telesford KM, Ochoa-Repáraz J, Christy M, Kasper EJ, Kasper DL, Robson SC, Kasper LH. A commensal bacterial product elicits and modulates migratory capacity of CD39 + CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes. 2014;5(4):552–561. doi: 10.4161/gmic.29797 [DOI] [PubMed] [Google Scholar]
- 84.Wang Y, Telesford KM, Ochoa-Repáraz J, Haque-Begum S, Christy M, Kasper EJ, Wang L, Wu Y, Robson SC, Kasper DL, Kasper LH. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat Commun. 2014;5(1):4432. doi: 10.1038/ncomms5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rolak LA. Multiple sclerosis: it’s not the disease you thought it was. Clin Med Res. 2003;1(1):57–60. doi: 10.3121/cmr.1.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palumbo S, Pellegrini S. Experimental in vivo models of multiple sclerosis: state of the art. In: Zagon IS, McLaughlin PJ, editors. Multiple Sclerosis: perspectives in Treatment and Pathogenesis. Brisbane (AU: ): Codon Publications; 2017. p. 173–183. http://www.ncbi.nlm.nih.gov/books/NBK 470145 [PubMed] [Google Scholar]
- 87.Torkildsen Ø, Brunborg LA, Myhr K-M, Bø L. The cuprizone model for demyelination. Acta Neurol Scand. 2008;117(s188):72–76. doi: 10.1111/j.1600-0404.2008.01036.x. [DOI] [PubMed] [Google Scholar]
- 88.Dal Canto MC, Kim BS, Miller SD, Melvold RW. Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelination: a model for human multiple sclerosis. Methods. 1996;10(3):453–461. doi: 10.1006/meth.1996.0123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


