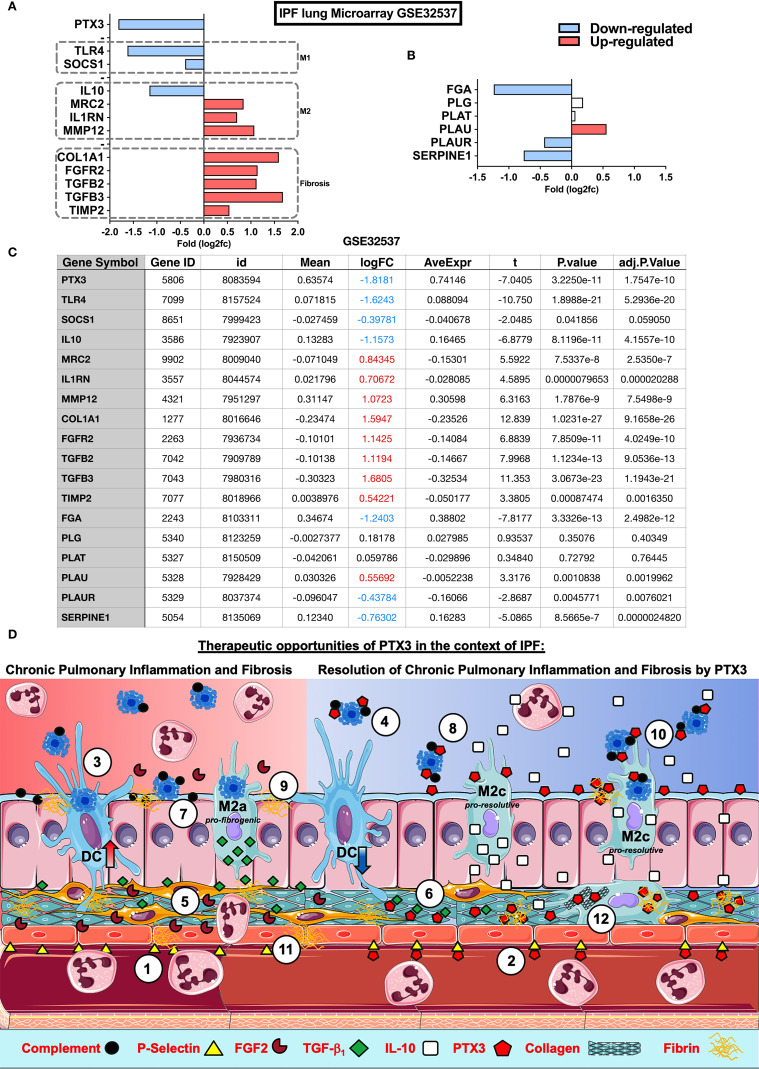
Figure 2.
Impact of PTX3 in Idiopathic Pulmonary Fibrosis and therapeutic opportunities. Microarray analysis of lung samples from IPF (n=119) and healthy (n=50) individuals from GEO database: GSE32537 were analyzed by Phantasus (58) (https://genome.ifmo.ru/phantasus). (A) Lung expression of PTX3, fibrogenic markers as COL1A1, FGFR2, TGFB2, TGFB3 and TIMP-2, M1 (SOCS1 and TLR4) and M2 (IL-10, MRC2, IL1RN, and MMP-12) macrophage polarization markers (B) Expression of coagulation cascade FGA (Fibrinogen alpha-chain precursor), PLG (Plasminogen precursor), PLAT (Tissue-type Plasminogen Activator precursor), PLAU (Urokinase-type Plasminogen Activator precursor), PLAUR (Urokinase Plasminogen Activator Receptor) and SERPINE1 (PAI-1 Plasminogen Activator Inhibitor-1) in IPF and health samples. (C) Data table with analysis from GEO database GSE32537 analyzed by Phantasus according to the instructions for the use of the application. Differences were considered significant at P value <0,05. (D) Possible mechanisms and therapeutic opportunities of PTX3 in the context of IPF. IPF is characterized by a reduced PTX3 production (red background), however PTX3 may act as an anti-inflammatory as well as a pro-resolutive modulator of chronic pulmonary inflammation and fibrosis in IPF (blue background) at different levels: (1) Neutrophil influx is facilitated through the interaction with P-selectin expressed on the surface of ECs, (2) PTX3 could antagonize endothelial P-selectin, dampening neutrophil influx during chronic pulmonary inflammation; (3) the abundance of apoptotic cells in airways from IPF is related to DC phagocytosis and activation that sustain chronic lung inflammation, (4) PTX3 may block apoptotic cell internalization and consequent inflammation; (5) FGF2 activates fibroblasts and ECs, (6) however PTX3 interacts with FGF2 reducing its availability for binding to FGFR2 on fibroblasts and consequent fibrosis; (7) complement and apoptotic cell deposition in the lungs lead to chronic inflammation, (8) on the other side PTX3 may act as a scavenger preventing the excessive deposition of both complement components and apoptotic cells in lungs and consequent attenuation of tissue damage and inflammation; (9) Alveolar macrophages from IPF display defective efferocytosis and increased TGF-β1 production, contributing to tissue fibrogenesis, (10) while PTX3 may enhance macrophage efferocytosis and M2 polarization and resolution of inflammation by IL-10; (11) Finally, defective PTX3 production in IPF may increase fibrin deposition and fibrosis, (12) but PTX3 could contribute to the resolution of fibrosis, interacting with fibrin-clots and disorganized collagen fibers in the lung parenchyma, supporting fibrinolysis and clearance of ECM debris by macrophage phagocytosis, promoting lung tissue healing and repair.