FIGURE 3.
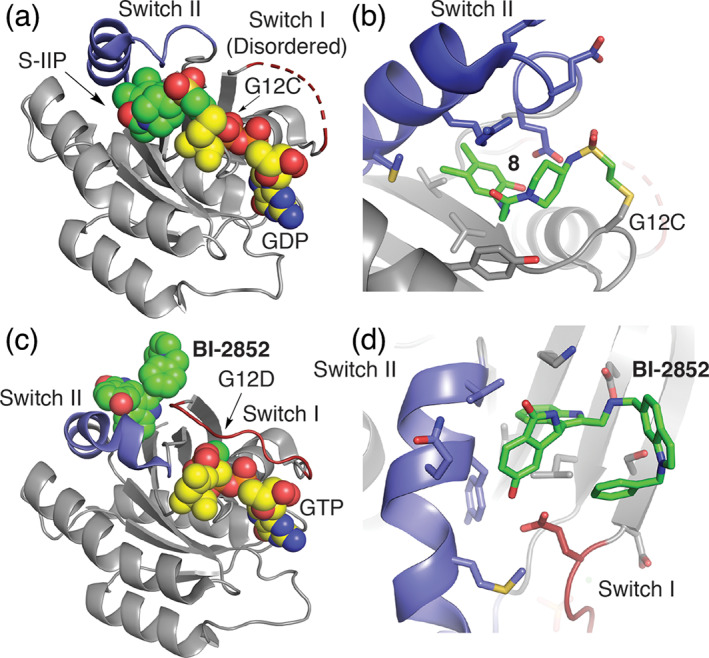
Binding modes of inhibitors in complex with mutant K‐Ras. (a) Co‐crystal structure of a K‐Ras C12‐targeting covalent inhibitor (PDB ID 4LYF 8 ) and (b) zoom in on 8 binding pose. 63 K‐Ras inhibitor forms a covalent bond with the mutant residue G12C cysteine residue and anchors the molecule within a previously unappreciated allosteric binding pocket underneath the Switch II (SII‐P, blue). (c) BI‐2852 based on PDB ID 6JG7 bond to the SI/II pocket of G12D with bound GTP and (d) zoom in on SI/II site. 64 The BI‐2852 covalent drug binds an allosteric site spanning structural elements of Switch I and II that is unchanged in active and inactive states of K‐Ras. GTP or GDP substrate sites are shown in spacefill with yellow carbon atoms
