FIGURE 6.
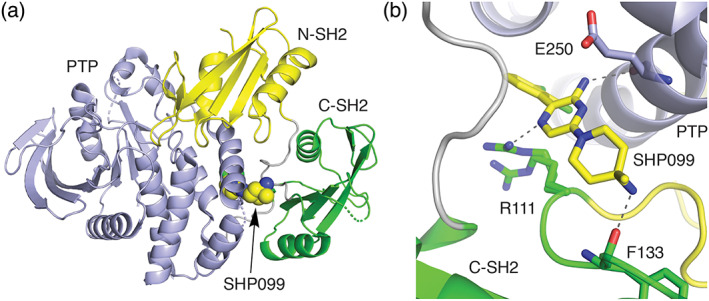
(a) Structure of autoinhibited SHP2 and (b) detail of the binding mode of SHP099 (PDB ID 5EHR). 122 In the inactive state, the tandem SH2 domains of SHP2 bind to the tyrosine phosphatase domain (blue) and the N‐terminal SH2 domain (yellow) directly blocks the phosphatase active site. Allosteric inhibitor SHP099 binds in pocket between the C‐terminal SH2 domain (green) and the phosphatase domain, acting as a molecular glue to stabilize the inhibitory interactions of the SH2 domains
