Figure 1.
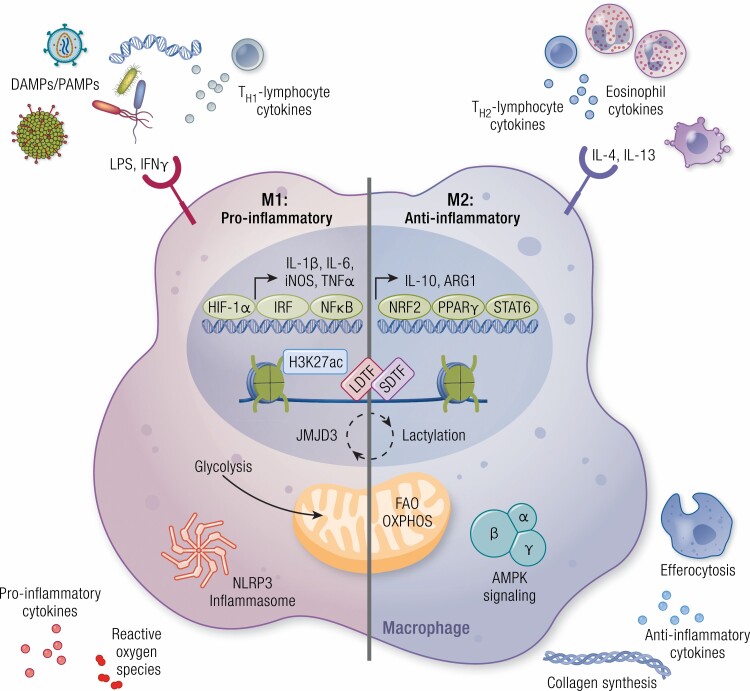
Extracellular cues from the environment polarize macrophages. Macrophages express a variety of cell surface receptors that sense extracellular factors that induce macrophage polarization toward a proinflammatory (M1) or anti-inflammatory (M2) state. In cultures, proinflammatory macrophages are polarized by stimulation with lipopolysaccharide (LPS) and interferon γ (IFN-γ). The proinflammatory phenotype of macrophages can also be stimulated by detection of danger-associated molecular patterns (DAMPs)/pattern-associated molecular patterns (PAMPs), such as bacteria, viruses, and exogenous DNA, as well as by cytokines produced by TH1-lymphocytes. Polarization is in part dictated by the activation of transcription factors, such as hypoxia-inducible factor-1α (HIF-1α), IFN-regulatory factor (IRF), and nuclear factor κB (NFκB), and the induction of their proinflammatory gene networks. Furthermore, metabolic reprogramming occurs in these macrophages, primarily by the upregulation of glycolysis. Together these transcriptional and metabolic changes result in inflammasome activation and the production of proinflammatory cytokines and reactive oxygen species. Anti-inflammatory macrophages are produced by the treatment of macrophages with interleukin-4 (IL-4) or IL-13 in vitro and in vivo signals are received from eosinophils and TH2-lymphocytes. Transcription factors required for M2-like polarization include nuclear factor erythroid 2-related factor 2 (NRF2), peroxisome proliferator-activated receptor γ (PPARγ), and signal transducer and activator of transcription 6 (STAT6), leading to the production of anti-inflammatory genes such as IL-10 and arginase 1 (ARG1). In these anti-inflammatory macrophages, metabolism is shifted toward fatty acid oxidation (FAO) and oxidative phosphorylation (OXPHOS). Here, adenosine 5′-monophosphate–activated protein kinase (AMPK) signaling is promoted, and in turn leads to the production of anti-inflammatory cytokines, collagen synthesis, and efferocytosis. Epigenetic modifications also play a role in determining macrophage identity and function, including chromatin remodeling such as acetylation of lysine 27 of histone 3 (H3K27ac) accompanied by binding of transcription factors (LDTF, lineage-determining transcription factors; SDTF, signal-dependent transcription factors), an epigenetic signature that is strongly influenced by environment-derived factors. The plasticity of macrophages between its proinflammatory and anti-inflammatory polarized states can be induced by feedback pathways engaged by the metabolic programs that involve epigenetic changes, such as histone lactylation or demethylation (JMJD3).