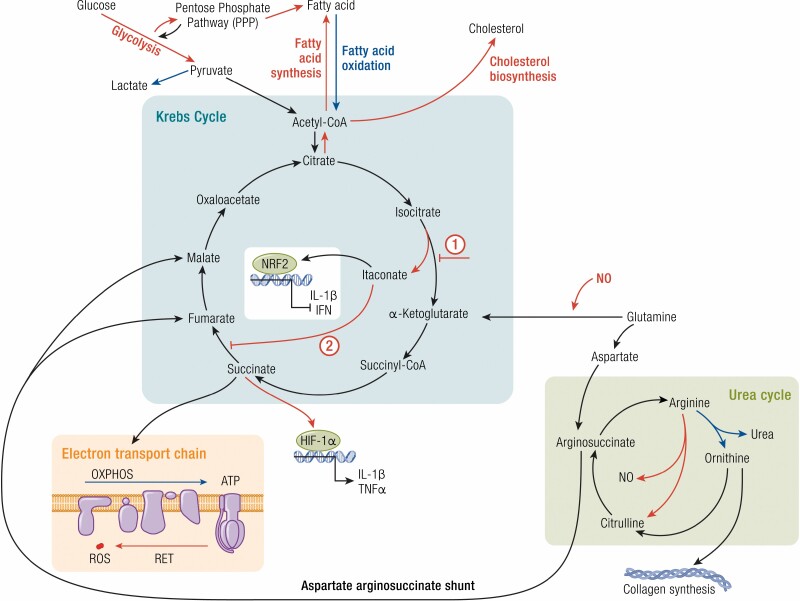
Figure 2.
Macrophage metabolism influences their function. Metabolic changes in macrophages primarily involve alterations to the Krebs cycle. Proinflammatory macrophages (red) and anti-inflammatory macrophages (blue) favor specific pathways that influence their function or inflammatory state. Proinflammatory macrophages favor glycolysis and fatty acid and cholesterol synthesis pathways, whereas anti-inflammatory macrophages prefer fatty acid oxidation. Within the Krebs cycle proinflammatory macrophages exhibit 2 key break points: (1) the inhibition of isocitrate conversion to α-ketoglutarate (by isocitrate dehydrogenase) and the production of itaconate and (2) the inhibition of succinate dehydrogenase, which catalyzes the conversion of succinate to fumarate. This accumulation of succinate allows for the stabilization of the hypoxia-inducible factor-1α (HIF-1α) and the transcription of proinflammatory genes. Succinate metabolism fuels the electron transport chain, which is important for promoting oxidative phosphorylation (OXPHOS) in anti-inflammatory macrophages. However dysfunction in the electron transport chain, including reverse electron transport (RET), produces reactive oxygen species (ROS), characteristic of proinflammatory macrophages. In the urea cycle, arginine metabolism by arginase-1, a prototypical anti-inflammatory marker, produces ornithine, which is a precursor for collagen synthesis used by these macrophages to promote tissue repair and wound healing. Proinflammatory macrophages, on the other hand, express inducible nitric oxide synthase (iNOS), which metabolizes arginine to produce citrulline and nitric oxide (NO), further enhancing the inflammatory status of these macrophages.