Figure 1. Reactive Astrocytes in Mouse and Humans in the Context of Central Nervous System (CNS) Viral Infection.
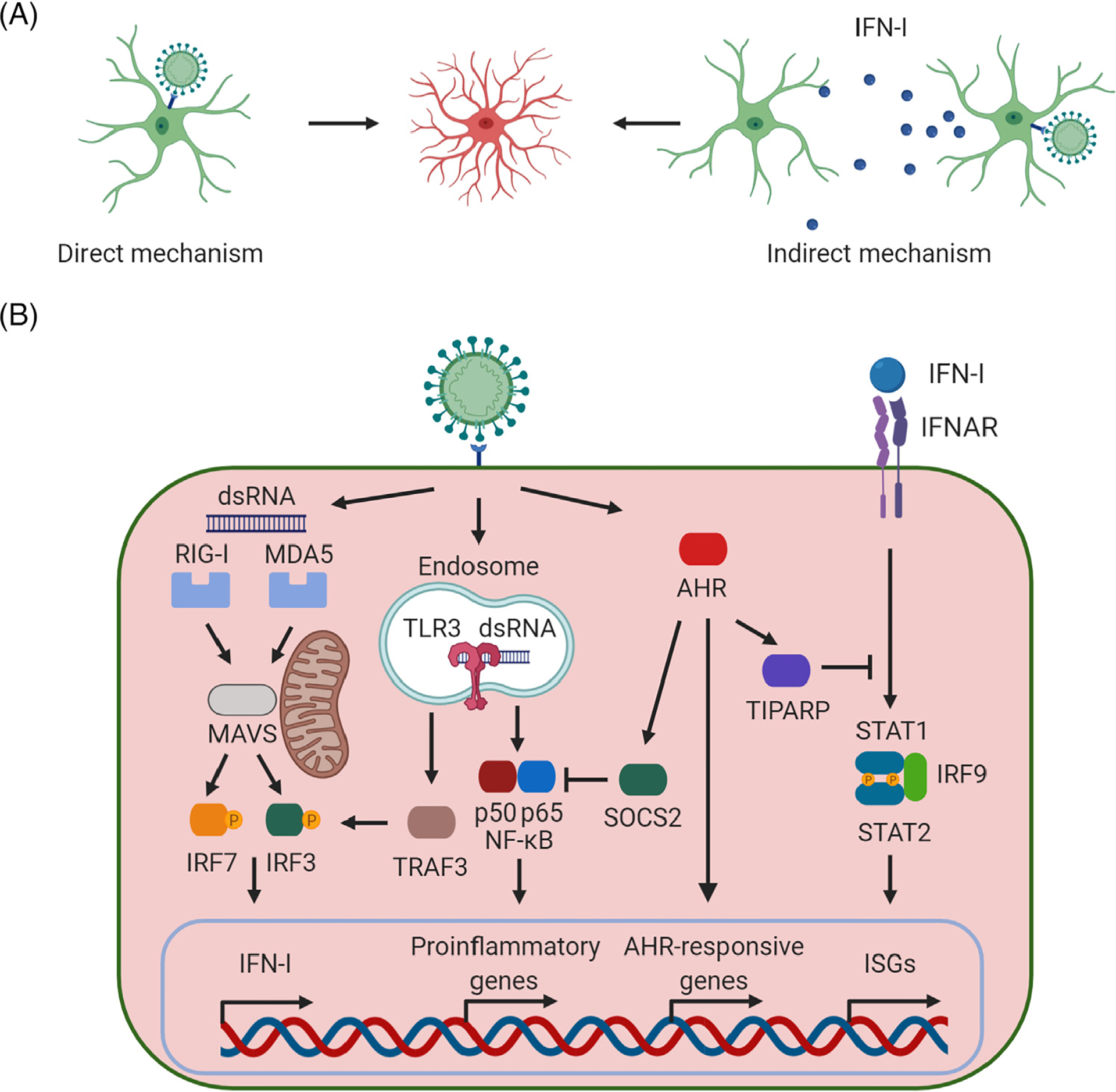
(A) Reactive astrocytes are induced in the context of viral infections through two major mechanisms: direct and indirect. The direct mechanism involves infection of astrocytes, which then become reactive astrocytes. Conversely, the indirect mechanism involves signaling molecules [e.g., interferon type I (IFN-I)] produced by neighboring infected cells (including other astrocytes as well as other cell types in the CNS). (B) Major signaling pathways involved in the generation of reactive astrocytes on viral infection. Double-stranded RNA (dsRNA), one of the major viral pathogen-associated molecular patterns (PAMPs), is recognized by the endosomal Toll-like receptor 3 (TLR3) as well as the cytoplasmic retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated protein 5 (MDA-5), leading to the synthesis and release of IFN-I. Then, secreted IFN-Is activate autocrine and paracrine signaling cascades through the IFN-I receptor inducing the expression of IFN-stimulated genes (ISGs), mounting an effective antiviral response [32–35]. AHR, aryl hydrocarbon receptor; IFNAR, interferon-α/β receptor; IRF3, interferon regulatory factor 3; IRF7, interferon regulatory factor 7; IRF9, interferon regulatory factor 9; MAVS, mitochondrial antiviral signaling protein; NF-κB, nuclear factor kappalight-chain-enhancer of activated B cells; SOCS2, suppressor of cytokine signaling 2; STAT, signal transducer and activator of transcription; TIPARP, TCDD inducible poly(ADP-ribose) polymerase; TRAF3, tumor necrosis factor receptorassociated factor 3. This figure was created using BioRender (https://biorender.com/).
