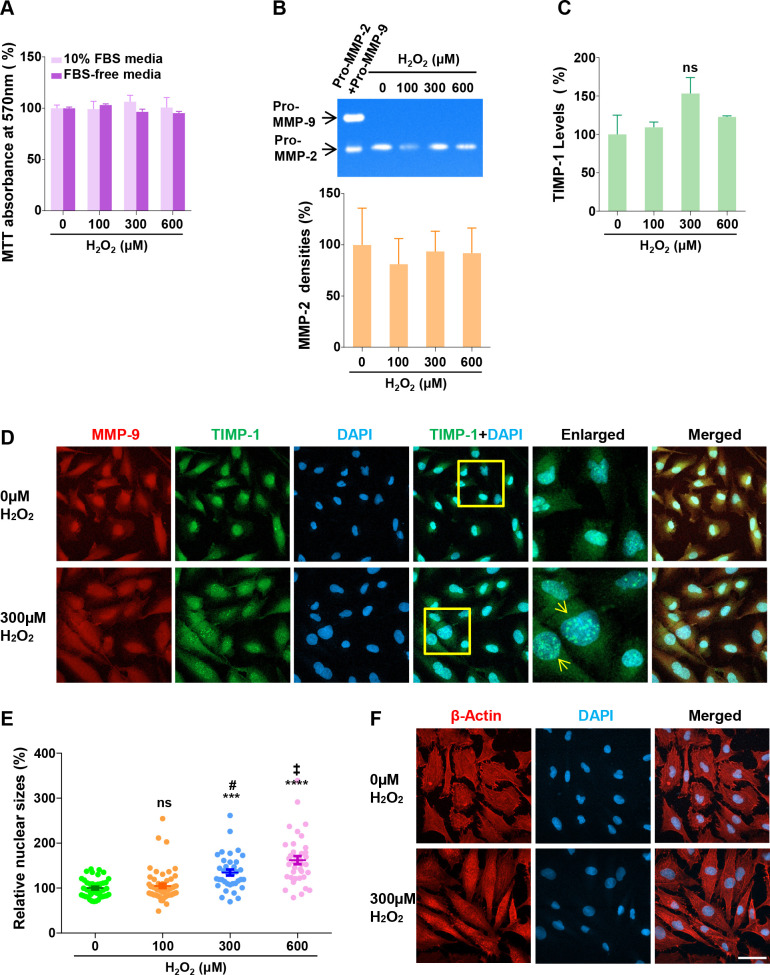
Fig 4. Effect of oxidative stress on intracellular MMP-9 and TIMP-1.
(A) MIO-M1 cells were cultured in the presence of H2O2 at indicated concentrations in the regular (10% FBS) or FBS-free media, for 24 h, and then subjected to MTT assay to measure viable cell densities. Relative cell densities are presented as % mean ± SE (n = 3), with control (0μM) set as 100%. (B) The cells were treated in the same way as in (A), and then CMs were collected, to perform gelatin zymography and ELISA assay. (Top) the CMs of the same volume from each treatment was subjected to gelatin zymography. One representative zymogram is shown to visualize secreted MMPs. A mixture of recombinant human pro-MMP-2 and pro-MMP-9 (Pro-MMP-2+Pro-MMP-9) were applied to the gel prior to electrophoresis as a MMPs marker control. (Bottom) The density of each MMP band was quantified using Image J software (n = 3). The individual values of MMP band densities were normalized by the corresponding MTT absorbance values. (C) Secreted TIMP-1 levels in the same CMs were measured using TIMP-1 ELISA assay (n = 3). The individual ELISA values were normalized by the corresponding MTT absorbance values. ns, not significant (p>0.05, vs. 0μM). (D) MIO-M1 cells were cultured in the presence of 300 μM H2O2 for 24 h, and then subjected to IHC confocal microscopy, for dual IHC staining with MMP-9 (red) and TIMP-1 (red) antibodies. Insets in TIMP-1+DAPI micrograms are enlarged, and arrows point to the bigger nuclei with increased TIMP-1 speckles. (E) Nuclear sizes in the micrograms of the cells treated with increasing concentrations of H2O2 were measured, using Image J software. Relative nuclear sizes are presented as % mean ± SE. ns, not significant (p>0.05 vs. 0μM); ***, p<0.001 (vs. vs. 0μM; ****, p<0.0001 (vs. 0μM); #, p<0.001 (vs. 100μM); ‡, p<0.01 (vs. 300μM). The numbers of randomly selected nuclei measured were 43 (0μM), 55 (100μM), 36 (300μM), and 37 (600μM). (F) β-actin (red) antibody was used to perform a confocal microscopy after 300μM H2O2 treatment for 24h. Representative micrographs of untreated (0μM) and treated (300μM) groups are presented. bars, 50 μm.