Figure 5.
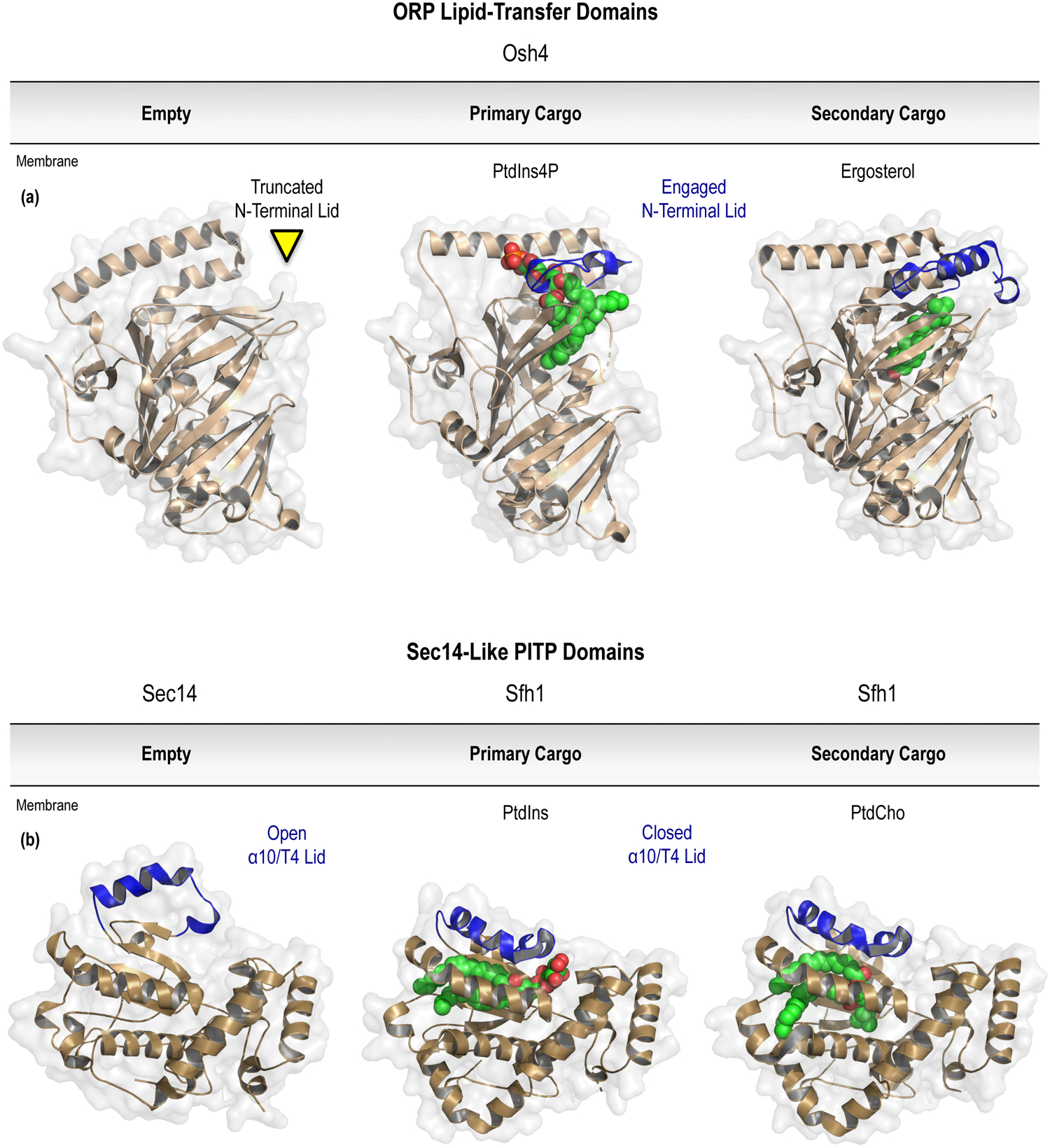
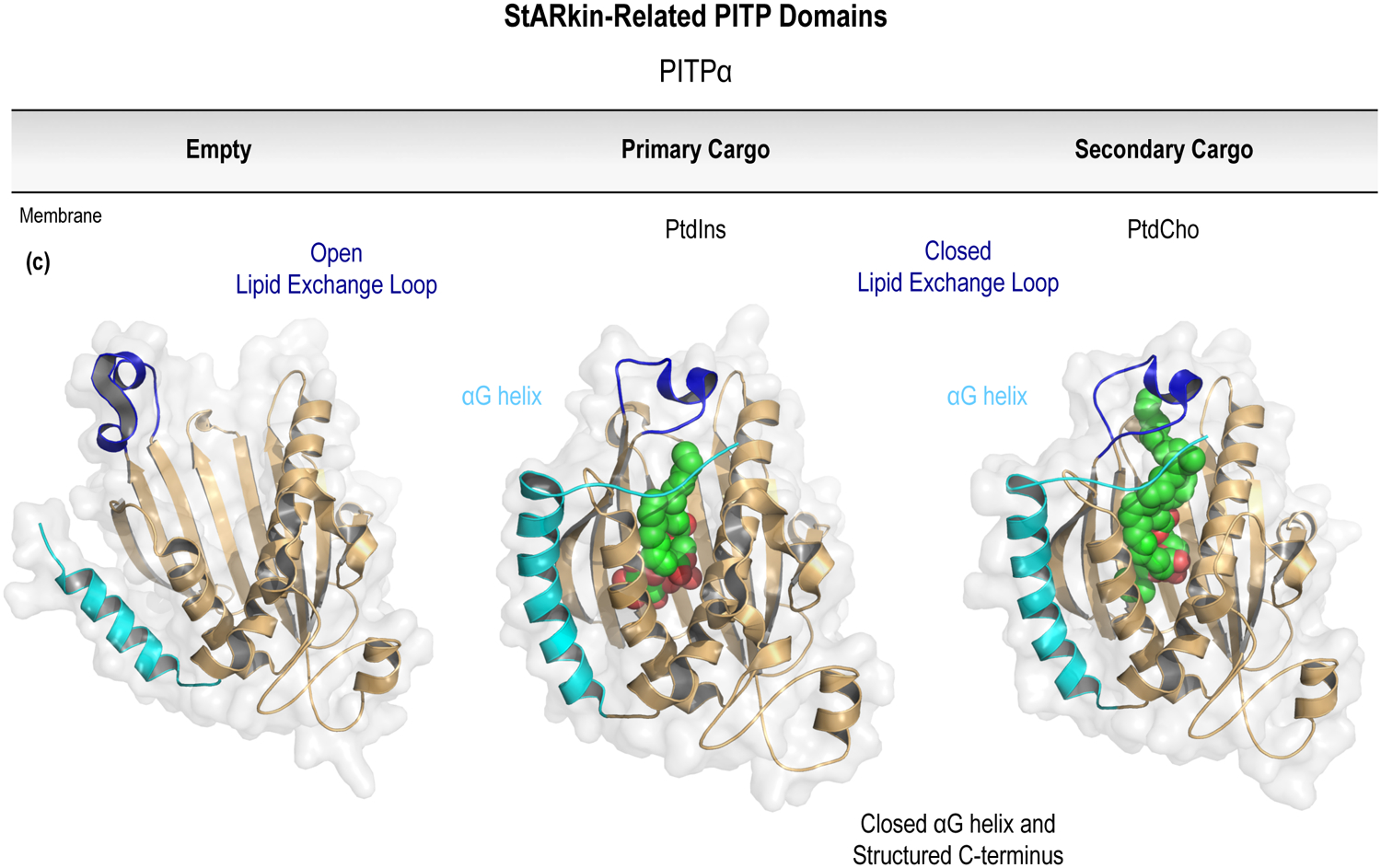
Lipid-transfer domains with selectivity for PPIn species. For each of the known families of PPIn-coordinating LTPs, the structure of the open LTD fold is shown in relationship to structures with either the primary or secondary lipid cargo bound. Of note, each of the LTPs are shown with the opening of the LTD pocket located at the top of the molecule. Unlike the ORP or Sec14-like LTDs, the PPIn headgroup is buried within the domain, with the fatty acyl chains projecting upwards to the top of the binding pocket. (a) The well-studied family of ORPs are important regulators of non-vesicular lipid transport across eukaryotes; including a conserved function for transporting PtdIns4P (PDB entry 3SPW) and a variety of secondary lipid cargoes, including sterols (PDB entry 1ZHZ). Please note that the open fold (PDB entry 1ZI7) could only be crystallized following truncation of the N-terminal lid (shown in blue) that gates the hydrophobic lipid-binding pocket. (b) The large family of Sec14-like PITPs might play more diverse roles outside of lipid transport to control intracellular signaling responses. The recognition of the conserved PtdIns (PDB entry 3B7N) and PtdCho (PDB entry 3B7Q) cargoes clearly involves unique binding surfaces within the PITP, as well as the dynamic reorganization of the α10/T4 lid (highlighted in blue) relative to the unbound structure (PDB entry 1AUA). (c) The prototypical StARkin-related PITP domain is also shown bound to PtdIns (PDB entry 1UW5) and PtdCho (PDB entry 1T27). Compared to the open fold (PDB entry 1KCM), closure of the lipid exchange loop (shown in blue) also stabilizes the elongated C-terminus to pin the αG helix (cyan) in the closed conformation. For further details, please refer to Sections 7.1 (ORPs) and 7.2 (7.2.1, Sec14-like; 7.2.2, StARkin-related) of the text. Prepared using the PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.
