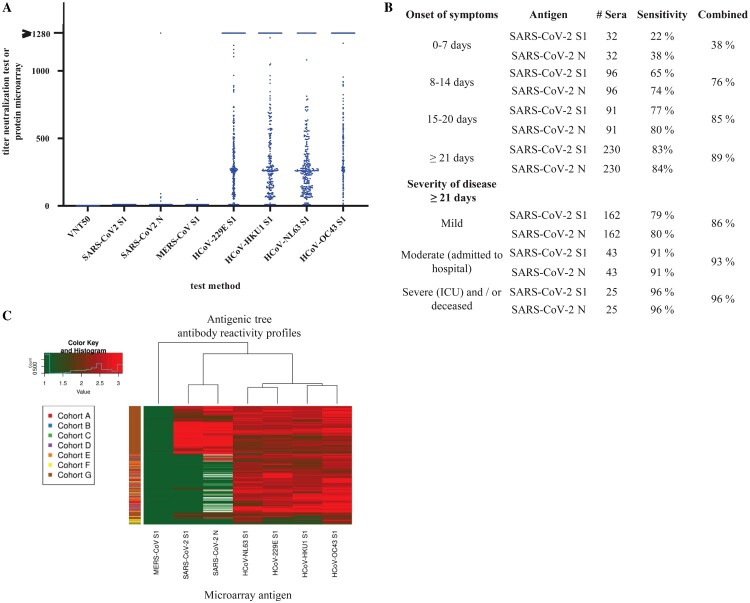
Figure 1.
Validation of SARS-CoV-2 protein micro-array. (A) Specificity of SARS-CoV-2 VNT50 and SARS-CoV-2 S1 and N antigens on the HCoV-PMA measured with pre-COVID-19 sera from different cohorts that were sampled between 2011 and 2019. (B) Sensitivity of SARS-CoV-2 S1 and N antigen protein micro-array determined with 449 sera of 330 RT-PCR-confirmed SARS-CoV-2 cases, analysed by days post onset symptoms and severity of disease: i.e. mild (no admission to hospital), moderate (admission to hospital, but not ICU) and severe (admitted to ICU and/or deceased). (C) Heatmap displaying log10-transformed micro-array titres of 255 pre-COVID-19 cohorts sera, and sera of 449 RT-PCR-confirmed SARS-CoV-2 cases against antigens of all currently circulating coronaviruses (colour key of titres indicated in the top left corner: green – negative/low titres, red – high titres, white – not done). The cohorts are: (A) healthy blood donors, (B) acute cytomegalovirus patients, (C) acute Epstein–Barr virus patients, (D) patients with recent PCR-confirmed seasonal HCoV infection, (E) patients with recent non-coronavirus influenza-like-illness infection, (F) patients with respiratory complaints of unknown aetiology and (G) cases with RT-PCR-confirmed SARS-CoV-2 infection. All sera from cohorts A-F were sampled between 2011 and 2019.