Fig. 1. Simplified schematic of A) sources of ROS and B) ROS detoxification by the antioxidant defense system.
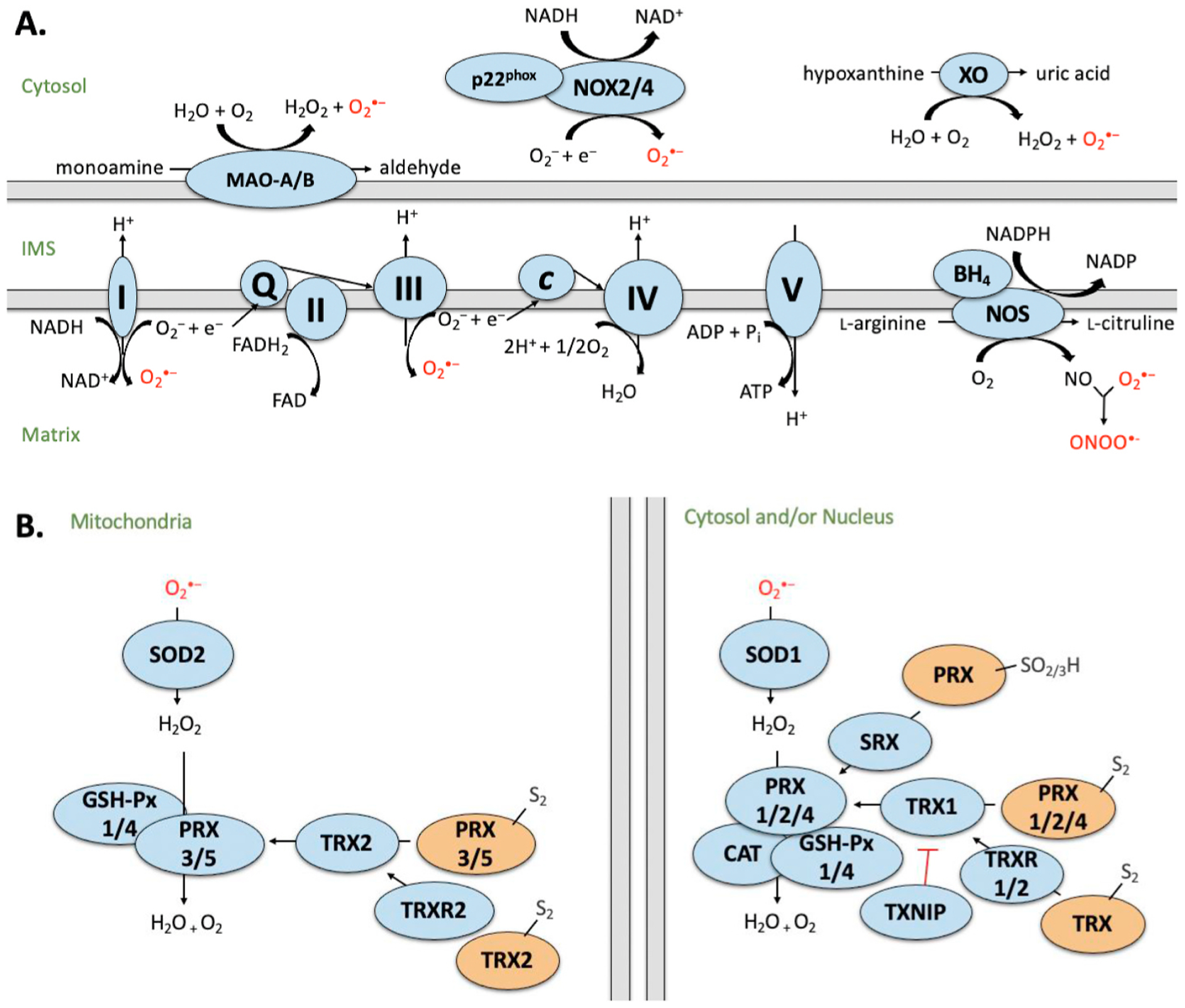
Sources of ROS generation in the cardiomyocyte including mitochondrial electron transport chain (ETC), monoamine oxidases (MAO), calpains, nicotinamide adenine dinucleotide (NADH) oxidases (NOX), xanthine oxidase (XO) and uncoupled nitric oxide synthase (NOS). Complex I (NADH dehydrogenase) transfers electrons from NADH and passes to ubiquinone (Q). Complex II (succinate dehydrogenase; SDH) donates electrons from succinate (as part of the Krebs cycle) to Q via FeS clusters. Complex III (cytochrome c oxidoreductase) transfers the electrons carried by ubiquinol (QH2) to cytochrome c (Cyt c). Complex IV (cytochrome c oxidase) transfers electrons (e−) from cytochrome c to O2 to generate water (H2O). Complex V (F1F0 ATP synthase) exchanges protons from the IMS to the matrix, which drives phosphorylation of ADP to form ATP. MAO generates H2O2 and reactive aldehydes as by-products during the oxidation of monoamines. NOX forms a heterodimer with p22phox and produces upon electron transfer from NADPH to molecular O2. XO reacts with molecular oxygen to produce and H2O2 during the production of uric acid. NOS consumes NADPH and O2 to convert L-arginine to L-citrulline, generating NO and NADP as by-products. I–V: Denotes ETC complex number. B) Generation of ROS is counterbalanced by the antioxidant defense system. Superoxide is converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). H2O2 is converted to H2O by glutathione peroxidase (GSH-Px), peroxiredoxin (PRX) or catalase (CAT). Activity of PRX is regulated by sulfiredoxin (SRX) and thioredoxin (TRX), which is further regulated by TRX reductase (TRXR) and its endogenous inhibitor, thioredoxin interacting protein (TXNIP). Blue indicates active state. Orange indicates inactive state. Intermembrane space (IMS). Proteins have been compartmentalized according to their predominant intracellular localization.
