Abstract
Long is the way and hard, that out of COVID-19 leads up to light. The virus is highly contagious and spread rapidly and the number of infections increases exponentially. The colossal number of infections and presence of the novel coronavirus RNA in human wastes (e.g. Excreta/urine) even after the patients recovered and the RT-PCR tests were negative, results in massive load of the viral in water environments. Numerous studies reported the presence of SARS-CoV-2 in wastewater samples. The risk of contaminating water bodies in the regions which suffer from the lack of proper sanitation system and wastewater treatment plants (mostly in developing countries) is higher. Since solar water disinfection (SODIS) is usually used by people in developing countries, there is a concern about using this method during the pandemic. Because the SARS-CoV-2 can be eliminated by high temperature (>56 °C) and UVC wavelength (100–280 nm) while SODIS systems mainly work at lower temperature (<45 °C) and use the available UVA (315–400 nm). Thus, during a situation like the ongoing pandemic using SODIS method for wastewater treatment (or providing drinking water) is not a reliable method. It should be reminded that the main aim of the present study is not just to give insights about the possibilities and risks of using SODIS during the ongoing pandemic but it has broader prospect for any future outbreak/pandemic that results in biological contamination of water bodies. Nevertheless, some experimental studies seem to be necessary by all researchers under conditions similar to developing countries.
Keywords: Solar disinfection (SODIS), Water treatment, UV disinfection, Developing countries, Novel coronavirus, Contaminated water
Graphical abstract
1. Introduction
“COVID-19 is more than a health crisis. Millions have lost their jobs. The global economy is headed for the sharpest contraction since the Great Depression. The political, economic and social effect of the pandemic will be felt for years to come. We should be all in this together”
Dr. Tedros Adhanom (Director-General of the World Health Organization)
The world was surprised at the end of 2019 with shocking news about the advent of a new virus from the family of Coronaviridae [1]. Although the beginning of severe acute respiratory syndrome 2 (SARS-CoV-2) firstly was reported by Chinese officials at the end days of 2019 and in more than 2000 papers, researchers mentioned the city of Wuhan, in their abstract as the starting point of the pandemic [2], but there are some pieces of concrete evidence about the presence of the SARS-CoV-2 in different locations long time before the Chinese announcement. Such striking instances has created many doubts about the main origination of the virus since the viral RNA was detected in wastewater samples of Spain [3] and Brazil [4], eleven and four months before the first case was confirmed by the officials of those countries respectively. This means that the exact origination of the pandemic is quite vague. The contradiction of these reports steps the crucial importance of water and wastewater bodies in detecting some kind of epidemic into the spotlight before it becomes to a catastrophic pandemic like the COVID-19. Since the beginning of the pandemic, the experts of wastewater-based epidemiology (WBE) have conducted numerous researches throughout the world to examine the presence of the SARS-CoV-2 in various wastewater samples [5]. The presence of the SARS-CoV-2 in various water bodies increases because of human excreta and urine that release into the aquatic environment through wastewater (mostly in developing countries) and wastewater treatment plants (WWTPs). However, there are various routes for transmitting the virus into the aquatic environment. While the dominant transmission route of the SARS-CoV-2 is mainly established via the respiratory system, but there are anxieties over the effects opening new windows for transmitting the virus when the infected gastrointestinal glandular implied the potential transmission via fecal-oral [6]. Newly, probability transmission of the SARS-CoV-2 through water media has raised many questions [7]. Furthermore, it was stated due to the heavy load of the viral concentration in water bodies during the ongoing pandemic, using solar water desalination system to provide drinking water by desalinating impure water has too many risks [8]. Therefore, any water-related system and facility can be encounter with contamination of the virus and it can become a possible route to spread the SARS-CoV-2.
1.1. The objective of the present study
The main objective of the present study is to examine the effectiveness of SODIS method during the ongoing pandemic by focusing on the two important parameters that the novel coronavirus and the SODIS system have in common. The review organized in several steps and it is recommended to be meticulously followed by readers to realize the main concept. In the first step, the general concept of the SODIS systems is briefly introduced. In the second step, some parameters that have an effect on the performance of SODIS such as the type of UV content, free radicals, etc. are presented. In the third step, the photo-reactivation process is discussed to show the UV damage on the pathogen's genome and the repair mechanism while application of SODIS for virus eliminating, virus structure, and mechanism and resistance of viral inactivation through UV and temperature in the fourth section is discussed. The paper is followed in the fifth section by focusing on the contamination of water bodies to justify the fact that the feed water for SODIS systems can be heavily polluted by human waste through wastewater and wastewater treatment plants which result in biological contamination. The sixth section brought the importance of the fifth section into the spotlight by validating the presence of SARS-CoV-2 RNA in wastewater, groundwater, surface water, and rivers that discussed in numerous studies. The seventh section is the most important part of this study because it discusses two important factors that the SARS-CoV-2 and SODIS systems are severely affected by, which are UV and temperature. In the first section (i.e. 7.1) vulnerability of the virus to temperature is presented while the second sub-section (i.e. 7.2) discusses on the effect of various types of UV on the viability of the virus. In the eighth section, the risks of using SODIS systems during the ongoing pandemic by considering the earlier discussions in the seventh section and focusing on the effect of UV and temperature are presented. Eventually, in the ninth section summaries, concluding remarks, and future studies are recommended. It should be noted that the reason for brought a comprehensive content about the effective parameters on the SODIS performance, damage and repair mechanism of pathogen's structure, discussing the pollution of water environments by pathogens, etc. is to show that the present study is not limited to the ongoing pandemic but it has broader prospect about any possibility for the future outbreak/pandemic that results to contaminate aquatic environment throughout the world.
2. Solar water disinfection
More than half of the world population (4 billion) is living in conditions that face with severe water scarcity at least one month of each year [9] while consuming contaminated water from available water resources by people lead to a high risk of waterborne diseases, especially in poor and rural communities. The importance of the providing safe drinking water can be elucidated when we consider that the UN in two of the most important action plans called MDGs and SDGs between 2000 and 2030 assigned a specific goal to water [10]. The importance of providing safe drinking water is more critical in low-income and developing countries because of poor sanitation networks and insufficient/improper/lack of wastewater treatment plants (WWTP). A well-known instance is Brazil which has one of the largest water resources in the world but due to the poor sanitation system and lack of proper WWTPs, many areas of the country have limited access to adequate drinkable water resources [11] and people confronted many problems through contaminated water. Solar-based systems such as solar stills and solar water disinfection (SODIS) are proper options for extremely remote regions as well as low-income and poor communities [[12], [13], [14], [15]]. The SODIS is a point-of-use water treatment method that has been used all over the world, especially in Asia, Latin America, and Africa [16]. Nearly 5,000,000 people in almost 50 countries through the world are using this method for their daily consumption, most of them are located in developing countries with limited access to a source of a safe drinking water [16]. In these countries, waterborne pathogens such as Escherichia coli, Salmonella, Vibrio cholera, Giardia, Cryptosporidium [17], etc. cause serious and even life-threatening diseases. The SODIS is known as a low-cost household water treatment and storage (HWTS) method by which pathogenic microorganisms are eliminated through solar radiation and mild temperature. It is drastically cheaper than filtration, chlorination, even than solar stills [12,17].
In this method, a transparent container is filled with contaminated water and exposed to direct sunlight for at least 6–8 h [17]. Usually, containers are chose from domestic plastic or glass bottles. Plastic bottles are used more frequently because they are cheaper and more resistant but they should be replaced every six months, because prolonged exposure to direct sunlight can cause to the leaching of plastic materials into water [17]. It is recommended to consume solar disinfected water within 24 h because of the possibility of post-exposure regrowth of pathogenic microorganisms [17]. The exact mechanism of the SODIS is still unclear, but it is obvious that damage to the DNA/protein increasing cell wall permeability have significant roles in the process. The sensitivity of pathogen, available UV content, reactive oxygen species (ROSs), water/ambient temperatures, and exposure time are the main factors in the germicidal effect of the SODIS method that some of them are discussed in the following.
2.1. Hydroxyl and dioxygen radicals generated by photons
Photosensitizers are endogenous or exogenous molecules that can produce ROSs by reducing oxygen when they are excited by light. ROSs have a role in physiological reactions as secondary messengers [18]. Under normal conditions, the balance between production and elimination of ROS is controlled by a scavenging system to maintain cellular homeostasis [19], but under oxidative stress conditions, excessive production of ROS can cause many problems for the cell. Enzymes such as Catalase (CAT) and Superoxide dismutase (SOD) provide cellular defense against ROS and protect cells from radical attacks [18]. These enzymes can be inactivated by photons [18], which has an important role in solar disinfection. ROSs can cause oxidative damage to DNA and other cell components [16,18,19]. It produces pyrimidine dimers and single-strand breaks (SSBs) which are lethal to the cell [18,20]. It can also affect cell membrane penetration by inducing oxidation reactions to hydroxyl groups of cell wall and cell membrane [21,22]. Typically, during the SOIDS process, two kinds of radicals are generated: hydroxyl radicals (•OH) and dioxygen radicals (1O2). The first one usually considers a powerful oxidant while the second one is a moderate oxidant. Generally, the predominant cause of pathogen annihilation (in the case of protozoa and bacteria) by hydroxyl radicals is damaging cell membrane through oxidizing lipid [23,24]. Since hydroxyl radicals are exceedingly reactive, they have a short lifetime and they are generated on-site by regular advanced oxidation process (AOP) by ultraviolet activation of hydrogen peroxide or ozone [25]. However, generating this kind of hydroxyl for small and poor communities has difficulties because of excessive demand for electrical energy and chemicals while other methods such as TiO2-based photocatalysis given the same results in the absence of aforementioned challenges [25]. Nevertheless, one of the well-known obstacles for hydroxyl radicals in water treatment is removing the generated •OH by natural organic matters (which is not an implausible phenomenon in wastewater), especially in complex water matrices which results in to drastically decline the effectiveness of the disinfection process [26]. Although, by combining stored hydrogen peroxide with harvested solar energy for photocatalytic synthesize to produce •OH; one of the obstacles (that is the constant presence of solar radiation to produce •OH) in solar-based water treatment systems is addressed, but this method also has the disadvantage of reducing singlet oxygen to hydrogen peroxide instead of H2O (or simply: catalytic selectivity). Furthermore, another method to produce •OH such as utilizing Fenton catalyst is still on laboratory scale and it applications under real conditions is not realized yet. Conversely, moderate oxidants such as singlet oxygen (1O2) is stable higher which means that they have less reactivity compared to powerful oxidants (•OH). In comparison to •OH radicals, they have a longer lifetime which means this kind of radicals can go farther distant from the site of origination. This is a huge advantage for the 1O2 radicals to remain effective in complex water matrices with a high natural organic matter, in contrast to •OH radicals that loss their effectiveness in complex water matrices due to a higher rate of reactivity [[26], [27], [28]]. While our focus in the present study is viruses, specifically, the SARS-CoV-2; we have not discussed the inactivation mechanism of bacteria via singlet oxygen. However, the inactivation mechanism of bacteria by 1O2 is extensively discussed before [29]. Singlet oxygen considers as a powerful disinfectant against enveloped viruses. When 1O2 is reacting with lipid, the fluidity of the membrane is decreases which results in an increase in the energy requisite for host binding, subsequently, impede the membrane bind to the host cell [30]. Conversely, the Non-enveloped virus inactivation mechanism is leaned by damaging the genomic and capsid which is not our concern in the present study. To reinforce the effectiveness of this method of disinfection (i.e. producing 1O2) using fullerene that leads to a high yield of 1O2 is also considered but such photosensitizers should be separated before the water is consumed by individuals. It should be noted that by separating photosensitizers, performance of the system is severely affected and diminished [[31], [32], [33]].
2.2. Effect of UV on the performance of SODIS
The UV considered as one of the powerful tools for inactivating biologically-contaminated water due to some advantages such as the absence of generating by-products during the disinfection process in contrast with other methods like chlorination. Briefly, UV types were categorized based on their wavelength as the A type (315–400 nm), the B type (280–315 nm), and the C type (100–280 nm) which abbreviated as the UVA, UVB, and UVC wavelengths respectively [16]. Optical inactivation of pathogens is practical when the solar intensity and available UV is strong enough to damage to the pathogen's structure. However, in the case of SODIS, we should not count only on the most available UV on the earth (which is UVA, 315–400 nm). There is a huge difference between UV germicide/microbicide irradiance and the UVA wavelength. The effective UV irradiance is directly damaged to the genome of pathogens while the effectual wavelength for this phenomenon is below 280 nm (somewhere in 260–265 nm which is the highest UV absorption of nucleic acid [34]), because photons of the UVA wavelength are considered as low-energy photon while high energy photons in UVC wavelength are strong enough to damage the genome of pathogens. In this regard, we cannot rely on the effectiveness of the UVA. Even by using solar reflectors to increase the UV irradiance (by collecting not only the direct UV but also the diffuse) on cloudy days the energy of photons would not change and the only difference is increasing the available UVA [35]. However, in recent years using lanthanide elements [36] to convert photons of visible light with lower energy into photons with higher energy in the UVC region is proposed as a solution, but application of these materials due to the high rate of reactivity and low quantum yield is remained on the laboratory scale.
2.3. Temperature contribution to effectiveness of the SODIS
Thermal contribution to the solar disinfection process is considerable but its importance varies in different seasons. The effect of thermal stress is significant above the optimum temperature for the growth of microorganisms, as the structure and activity of enzymes will be affected [22]. For instance, most of the fecal bacteria are mesophilic (withstanding between the temperature of 20–45 °C) [17]. This means that inactivation through the effect of temperature is more possible above this point (i.e. 45 °C) which means that below 45 °C, the effect of temperature on bacterial inactivation is negligible [37,38]. Thus, the removal of microorganisms through solar disinfection is severely limited in winter. The thermal effect depends on the volume and turbidity of water and also environmental conditions. Thermal stress facilitates the process of DNA damage and inhibits DNA repair mechanisms [16,39]. It increases the cell wall permeability [39], limits the enzymatic activities [17,22], and also leads to protein denaturation which all of them are lethal to cells. In temperatures beyond 45 °C, a synergy between thermal and optical inactivation is reported by many researchers [16,18,37,39] which improves the solar disinfection performance, but it is effective in specific cases under specific conditions.
Sichel et al. [40] examined the effect of environmental conditions for removing E. coli by the SODIS method. Their results showed that the temperature of water in the warmest months of the year (July) did not exceed 40 °C. McGuigan et al. [38] simulated the process of SODIS by considering the effect of temperature, turbidity, and optical irradiation. Findings revealed that for complete inactivation of pathogens, the water temperature should reach 55 °C, while it was reported that in field investigations under real conditions for several experiments, the temperature of water did not even reach 45 °C [41]. Rincon et al. showed that the temperature of water in SODIS with and without TiO2 photocatalyst from 32.6 °C and 36.6 °C reaches 39 °C and 38.6 °C respectively [35]. Moreover, under real conditions in winter and summer, the water temperature cannot further than 30 °C and it is in the range of 15–21 °C and 25–30 °C respectively [42]. It can be concluded that the temperature of water regarding the results of numerous experiments in cold seasons is always quite low whereas in warm seasons just in rare cases the temperature is higher than 45 °C.
2.4. Time of exposure
Time of exposure is another important factor in solar disinfection which is affected by environmental conditions such as wind speed, solar intensity, and ambient temperature [43] and it depends on the type of pathogen and its features. For instance, full sunshine can eliminate the Somatic Phage [44] and bovine rotaviruses in less than 3 h [45], but on the contrary the T2 Phage is not inactivated even after 8 h [16]. In another study, Enterococcus faecalis, Salmonella spp. and Staphylococcus aureus exposed to sunlight, and 4 log10 CFU/100 mL in 6 h were obtained, but as the exposure time increased to 8 h, the concentration reduced to below the detectable level [22]. In suitable conditions, most waterborne pathogenic bacteria such as E. coli are removable within 6 h, which makes many diseases preventable [16]. Table 1 gives the SODIS application for various types of pathogens considering the rate of reduction, time of exposure, and solar intensity.
Table 1.
SODIS application for various types of pathogens with respect to reduction, time of exposure, and solar intensity.
| Reduction | Time of expose | Radiation/wavelength | Type of pathogen | Name of pathogen | Reference |
|---|---|---|---|---|---|
| 4 log | 20 min | 1050+_10 W/m2 | Bacteria | Campylobacter jejuni | [46] |
| 4 log | 90 min | 1050+_10 W/m2 | Bacteria | Enteropathogenic Escherichia coli (EPEC) | [46] |
| 4 log | 45 min | 1050+_10 W/m2 | Bacteria | Staphylococcus epidermidis | [46] |
| 4 log | 150 min | 1050+_10 W/m2 | Bacteria | Yersinia enterocolitica | [46] |
| 3 log | 5 h | 2000 kJ/m2 | Bacteria | Escherichia coli | [45] |
| 4 log | 6 h | – | Bacteria | Salmonella spp. | [22] |
| 4 log | 6 h | – | Bacteria | Staphylococcus aureus | [22] |
| 6 log | 1.5 h | 42 mW/m2 | Bacteria | Shigella dysenteriae type 1 | [47] |
| 6 log | 6 h | – | Bacteria | Shigella flexneri | [47] |
| 1.7+_0.4 log | 8 h | 870 W/m2 | Bacteria | Bacillus subtilis | [48] |
| 99.9% | 1.8 h | 5000 kJ/m2 | Virus | Bovine Rotavirus | [45] |
| 3 log | Less than 3 h | 2000 kJ/m2 | Virus | Bacteriophage f2 | [45] |
| 99.9% | 1.8 h | 5000 kJ/m2 | Virus | Encephalomyocarditis virus (EMCV) | [45] |
| 4.4 log | 6 h | 850 W/m2 | Virus | Poliovirus | [49] |
| 0.25 log 0.17 log 0.41 log 2.16 log |
1 h 2 h 4 h 6 h |
550 W/m2 | Protozoa | Acanthamoeba castellanii | [50] |
| 3 log 3.59 log 3.59 log 3.59 log |
1 h 2 h 4 h 6 h |
550 W/m2 | Protozoa | Naegleria gruberi | [50] |
| 0.62 log 1.92 log 1.92 log 1.92 log |
1 h 2 h 4 h 6 h |
550 W/m2 | Protozoa | Entamoeba invadens | [50] |
| 0.94 log 1.96 log 1.96 log 1.96 log |
1 h 2 h 4 h 6 h |
550 W/m2 | Protozoa | Giardia lamblia | [50] |
| 0.02 log 0.07 log 0.15 log 0.32 log |
1 h 2 h 4 h 6 h |
550 W/m2 | Protozoa | Cryptosporidium parvum | [50] |
| 4.2+_0.2 log | 6 h | 870 W/m2 | Protozoa | Acanthamoeba polyphaga | [48] |
| 0.01 log 0.08 log 0.24 log 1.42 log |
1 h 2 h 4 h 6 h |
550 W/m2 | Helminth | Ascaris suum | [50] |
| 5.4+_0.2 log | 6 h | 870 W/m2 | Fungus | Candida albicans | [48] |
| 5.5+_0.2 log | 8 h | 870 W/m2 | Fungus | Fusarium solani | [48] |
| 5+_0.2 log | 28 h | 870 W/m2 | Bacteria | Pseudomonas aeruginosa | [48] |
| 1.1 log | 8 h | 20 kJ/m2 | Virus | Hepatitis A virus | [51] |
| 0.8 log | 8 h | 20 kJ/m2 | Virus | Murine norovirus (MNV-1) | [51] |
| 2.9 × 10−3 | – | 280 nm | Virus | MS2 | [52] |
| 1.4 × 10−2 | – | 280 nm | Virus | PhiX174 | [52] |
| 2.5 × 10−4 | – | 280 nm | Virus | Adenovirus | [52] |
3. Damage and repair mechanism in pathogens
Numerous parameters can be contributed to the inactivation of pathogens.
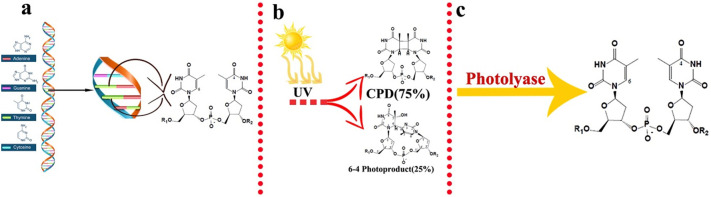
However, the most important parameters that can be affected the performance of the SODIS system and damaging DNA/RNA structure which is mentioned before as the ultraviolet irradiance, the temperature of water (thermal inactivation), various ROSs (i.e., hydroxyl and dioxygen radicals), and time of exposure. Ultraviolet radiation, ROSs, and thermal inactivation can, directly and indirectly, threaten cells by inducing DNA and protein damage. For instance, the irritation of DNA caused by direct UV, can cause a connection between two adjacent pyrimidine bases by covalent bonds and make pyrimidine dimers [16]. These dimers include Cyclobutane pyrimidine dimers (CPD) and 6-4 photoproducts, which are two of the most mutagenic DNA lesions [21,53]. CPDs are the most cytotoxic lesions which make up around 75% of UV-induced DNA damage products while 4-6 photoproducts are less abundant but they are more lethal [21]. Since it inhibited the progress of DNA polymerase [21], it can cause problems in DNA multiplication. Also, the RNA polymerases are stalling at these regions [21], thus transcription and translation may be eliminated. Hence, unrepaired DNA lesions can lead to mutation and inactivation of microorganisms [21]. Organisms have DNA repair mechanisms to remove DNA lesions and prevent their lethal effects. The photo-reactivation process (Fig. 1 ) is a common and simple mechanism for removing UV-induced DNA lesions, which is done by photolyase enzyme [21,54]. In the photo-reactivation process, pyrimidine dimers in DNA are recognized by photolyase under near-UV light (310–480 nm) [53]. Flavin adenine dinucleotide (FAD) and methyltetrahydrofolate (MTHF) are the two co-factors that are significant for this enzymatic function [54]. The MTHF absorbs energy and gets excited, then transduces an electron to FAD, subsequently, it reduces to FADH2. The FADH2 transfers the high-energy electron to the dimer and makes radicals. These pyrimidine dimers become highly unstable, so they separate into two pyrimidines and the DNA becomes fixed [55], so these repairs reduce the efficacy of UV disinfection [53].
Fig. 1.
Photo-reactivation repair mechanism of DNA. a) In normal condition, adjacent nucleotides within a single strand of DNA (ssDNA) are linked by a type of covalent bond, named phosphodiester bond, which is formed between the 5′ phosphate group of one nucleotide and the 3′-OH group of another. b) UV radiation leads two adjacent pyrimidine bases on one strand, become covalently fused, so cyclobutane pyrimidine dimers (CPDs) and 6-4 photoproducts are formed which can inhibit normal cellular function. (CPDs are composed when two vicinal pyrimidines joint through sharing two double bonded carbons. A covalent joint between a carbon at the 6 position of one pyrimidine ring and a carbon at the 4 position of the other one forms 6–4 photoproducts). c) In order to restore the integrity of DNA, Photolyase enzyme uses near-UV light to initiate electron transfer to break covalent bonds so that UV photoproducts become repaired.
4. Effectiveness of SODIS for virus removal
Among waterborne pathogens, viruses are usually more resistant to environmental inactivation [56] while their low infectious dose makes them more dangerous to the public health [57]. In the Section 2, we discussed about the important parameters on the SODIS performance. However, in the present study UV effect and thermal heating are the main factors in the SODIS process since these parameters are critical in inactivation the novel coronavirus. Among three types of UV, just the UVA wavelength and a small part of UVB reach on the surface of earth [21,58], and the UVC wavelength is completely absorbed by the Ozone layer. However, it should be reminded that the effective germicidal type of UV is the UVC wavelength and the UVA wavelength (which is the most abundant type of UV on the earth) has not a direct effect on genome's structure and its impact generally defined through the formation of ROSs [16,21]. As it discussed before, viruses like other pathogens can be damaged by the direct effect of UV on the genome and indirect endogenous or exogenous effect [59], (endogenous when the sensitizer molecules are a part of the virus itself, and exogenous when they are in water [51]) generally caused by ROSs [60,61]. By considering the simple structure of viruses, we can overlook the endogenous damage [59,62]. It was reported that F+ DNA coliphages were only sensitive to direct inactivation, while F+ RNA coliphages were also susceptible to indirect exogenous inactivation [52]. Human adenovirus (HAdV), human rotavirus (HRoV), PRD1, and MS2 are more susceptible to exogenous indirect inactivation; unlike poliovirus, porcine rotavirus, F+ DNA coliphages which are not much sensitive to exogenous sensitizers [52]. It should be mentioned that the most resistant viruses in the light-mediated inactivation are double-stranded DNA (dsDNA), double-stranded RNA (dsRNA), and single-stranded RNA (ssRNA) genome [63]. The reason for considering dsDNA viruses as the most resistant against radiation [52] is that they have more redundant genetic information as well as, ability to be repaired in the host cell [64] while in the case of RNA viruses, the resistance is because of the fact that they have uracil instead of thymine, and uracil dimer reactions have less quantum function than the corresponding thymine dimer reactions in DNA [[65], [66], [67]]. MS2 and adenovirus indicated similar inactivation rate, although adenovirus has a longer genome and absorbs more light. The reason is that adenovirus is a dsDNA virus, which can repair UV-induced damage on DNA [62]. Direct and indirect viral inactivation against MS2, phiX174, and adenovirus has been studied by Mattle et al. [62]. Findings revealed that phiX174 is the most susceptible one in direct inactivation, but the most resistant one in indirect inactivation through reactive species. It states that inactivation is mostly through direct process but O2 was also able to disinfect MS2 and adenovirus. The main photo-inactivation of viruses on the surface of earth, is in the range of UVB (280–320 nm) [59]. As we mentioned, most of the UVB wavelength cannot reach on the surface of earth which may be the reason of ineffectiveness of solar disinfection on viruses and it can even become worse when UV intensity decreases. For instance, coxsackievirus could be completely inactivated under simulated SODIS condition at optical irradiance 550 W/m2 within 2 h at 45 °C [50], while it was just partially inactivated by exposing it to the irradiance of 75 W/m2 for 6 h at maximum temperature of 34 °C [68]. Polo et al. experimentally examined the effectiveness of SODIS for eliminating the hepatitis A virus (HAV) and murine norovirus (MNV). Experiments conducted between 2 and 8 h exposure. Findings showed that around 0.81log10 and 1.1log10 reduction for hepatitis A virus (HAV) and murine norovirus (MNV) achieved respectively if time of exposure is 8 h [51]. Among studied viruses, HAdV and MS2 seem to be the most resistant viruses against environmental inactivation [52]. These results suggested that the UV content, temperature, and time are important parameters for an effective SODIS process. However, sunlight intensity (i.e., available UV content) is the most important factor in viral disinfection in comparison with temperature or time of exposure [51] because high temperature just can effectively harm capsid proteins but it's not much operative on the genome's structure [[69], [70], [71]]. In viruses, at temperatures above 40 °C, there is a thermal-optic synergy which means that as temperature increases, more viruses can be inactivated in exchange for each photon [59].
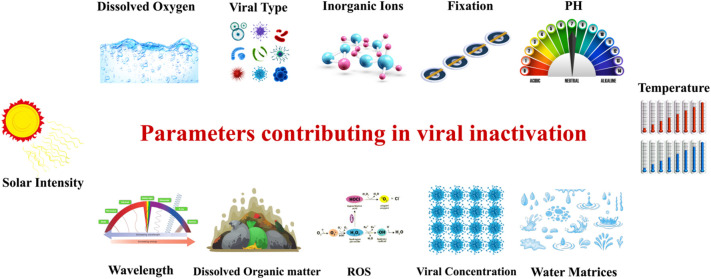
However, researchers concluded that complete viral disinfection through the SODIS process is not possible under natural conditions [57], therefore it needs more treatment time and even some interventions for total inactivation. Fig. 2 shows some of the most important parameters that have effect on viral inactivation.
Fig. 2.
Important parameters that can be affected the inactivation of viral. Crucial parameters that in most of SODIS researches were examined and discussed are solar intensity, temperature of water, fixation, and ROS.
5. Contamination of rivers, lakes, surface water, and groundwater
Nearly one-third of the world civilization is living next to the big rivers and their floodplains but in recent years anthropogenic barriers are a force to transboundary rivers and these regions results in many problems for occupants [72]. There are two important sources for pollution of water bodies in the globe which one of them is the human wastes [72]. Thus, contamination of water bodies via open drainage and wastewater is a common phenomenon throughout the world, especially in low-income and developing countries. For example, in Vietnam [73] the lack of WWTP leads to contamination of Sai Dong Nai rivers and groundwater which provide 1.2 million m3/day water of Ho Chi Minh City. In Kenya [74], open dumping of wastes in the absence of tight regulations results in heavy contamination of surface/groundwater while it was reported that in Nigeria, around 90% of hospital wastes without any treatment discharge into the surrounding environment [75]. In some of the African low-income countries such as Benin, Ghana, and Mali farmers utilized sludge and wastes as fertilizer by bribing to drivers of septic tanks [76,77] which is highly dangerous because some epidemic in the past returned to communities because of direct use of sludge [78,79]. In some of countries in South America between 60 to 70% wastewater left out to the water environments without treatment while almost forty percent of people in these regions are not linked to sewage systems [80]. For example, a big city like Quito with 3 million occupants in Ecuador treated only 3% of its wastewater [81]. Moreover, it was reported that around 75% of wastewater (45,000 L/s) in Mexico City drained to the environment without any formal treatment [82]. Some of the famous rivers in India such as the Ganges, the Gomti, and the Varanasi which are heavily polluted by direct discharge of human wastes and untreated wastewater, lead to many waterborne diseases [83,84]. Although WWTPs in industrialized and high-income countries are effective to prevent the contamination of water bodies by wastewater, but contamination by combined sewer flows (CSOs) is still inevitable in these countries [85]. It should be pointed out that, complete elimination of pathogens in WWTPs is not plausible because of inefficient WWTPs, high resistance of some pathogens, and high concentration of biological contamination in wastewater. While presence of pathogens in treated wastewater in high-income countries that have high standards for WWTP facilities is proved, the situation in low-income and developing countries most of which have not WWTP or effective water treatment facilities; is certainly worse than it can be imagined. It is worthy to be mentioned that, some well-known transboundary rivers and basins polluted by pathogens of wastewaters such as Amazon, Congo, Parana, Nile, Yenisey, Lena, Zambezi, Niger, Amur, Indus, Mekong, and Salween, to name a few [86]. In this regard, contamination of natural water bodies [87,88], rivers [89], groundwater [90], and freshwater environment [91] via the SARS-CoV-2 become to the one of main concerns of all researchers.
6. Presence of the SARS-CoV-2 in wastewater
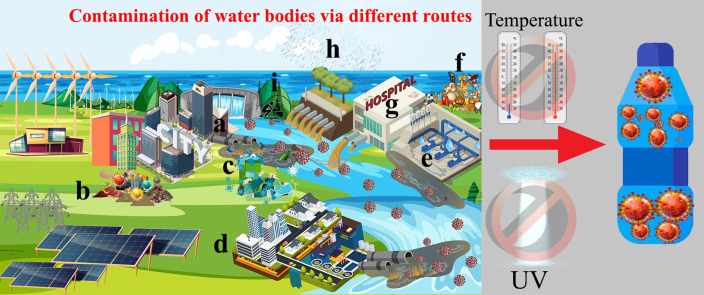
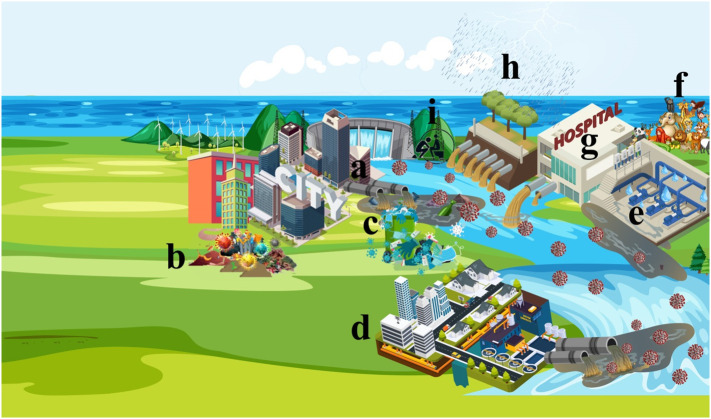
Presence of the SARS-CoV-2 in water bodies may occur via different routes. Some of these routes can be the main source of contamination such as wastewater drainage and direct discharging without treatment while some other routes like disposing personal protection equipment (PPE), in the environment (aquatic environment), open defecation, etc. are not consider as a major source of contamination (Fig. 3 ).
Fig. 3.
Possible routes of contamination of water bodies. Some routes such as wastewater discharge (a) and WWTPs have significant contribution on the contamination of water bodies while some routes such as secondary host (animals). Some routes such as open defecation and pit toilet mainly happened in developing countries and poor communities. The possible routes can be mentioned as: a) discharging cities wastewater, b) contaminated wastes, c) improper disposing personal protective equipment (PPE), d) wastewater treatment plants (WWTPs), e) sewer leakage, f) secondary hosts (animals), g) hospital sewage, h) combined sewer overflows (CSOs), i) open defecation and pit toilets.
Wastewater sewer network and open discharging are the main reasons that lead to entering pathogens (bacteria, virus, and protozoan) into the water bodies. In huge cities, a large amount of wastewater results in a high load of coronaviruses because of the population [92]. An efficient sewer system can dilute viral load, subsequently decreases the presence of the SARS-CoV-2 in an aquatic environment, but the large number of infected inhabitants by the virus in mega-cities increases the rate of viral load enters to the water bodies [92]. Presence of the SARS-CoV-2 in wastewater at the beginning of the pandemic was reported in different regions of the world [93]. Human urine and excreta consider as one of the main reasons which enter the SARS-CoV-2 in water bodies via sewer network. Researchers in numerous studies were detected presence of the SARS-CoV-2 in the stool of patients even though their respiratory tests are negative [6]. Further, it was reported that the virus in human feces based on the environmental conditions can be prolonged between 11 and 35 days [94]. This is highlighted the fact that if a patient is healed and recovered after infection by the virus, the feces that contain the viral RNA still can increase the load of the virus into the sewer network and subsequently increase the concentration of virion in wastewaters. However, it should be reminded that the exact infectivity of the SARS-CoV-2 in wastewater is not scrutinized yet [7]. Besides the lack of WWTPs and direct discharging of wastewater (like the city of Quito), the fast rate of infectious that results in a higher rate of infected persons waste entrance to the sewer network increases the risk of a heavy concentration of the virus in water bodies.
Among ten samples of secondary treated wastewater, the SARS-CoV-2 RNA is detected in 20% of the samples in Japan while the existence of the virus in 3 rivers is not confirmed which indicated that the low prevalence of the COVID-19 in the region results in the absence of virus RNA at those water bodies [95]. Also, it was disclosed, about 83% and 11% of untreated and secondary treated wastewater are contaminated by the COVID-19 virus. It was declared that around 65% of collected sewage samples during October 2019 to February 2020 are polluted by the SARS-CoV-2, suggesting the circulation of the virus in Italy months before starting the ongoing pandemic [96]. Experiments on 8 and 23 samples of treated and untreated wastewater from different sources in Paris confirmed the existence of the SARS-CoV-2 respectively [97]. Furthermore, presence of the novel coronavirus in treated wastewater of nine WWTPs stepped into the spotlight the fact that conventional WWTPs (such as active sludge) are ineffective for removing the virus in polluted water, suggesting advanced methods such as ozonation and membrane treatment [98]. It is important to notice that the SARS-CoV-2 is still detected in secondary treated wastewater of WWTPs which is commonly utilized by industrialized and high-income countries. Even though a high load of the SARS-CoV-2 in untreated wastewater (107 GC/L) and treated wastewater (removing 1–2 log10) is confirmed before, but significance or insignificance risks of the virus concentration on water bodies as well as transmission to the human is unclear yet. Presently, the minimum infectious dose (MID) of the SARS-CoV-2 (Which is the number of virion particles that result in infection) for humans is unknown [5]. Nevertheless, the expeditious dissemination of the disease suggests that the MID of the novel coronavirus is low, similar to other enveloped viruses [99]. In this regard, whether the concentration of the SARS-CoV-2 in water bodies is high risk or not, any possibility about transmission of the virus via water bodies should be considered to impede unexpected consequences [100].
7. Parameters influencing for inactivation of the SARS-CoV-2
During the COVID-19 pandemic numerous studies with different approaches and conditions for inactivating the novel coronavirus are performed. Castano et al. in a preprint categorized the inactivation methods of the SARS-CoV-2 to utilizing plasma, ozone, coating surfaces with prominent antimicrobial materials such as copper (Cu) and silver (Ag), heat treatment, UV irradiance, utilizing different chemical compounds [101]. Furthermore, other unconventional methods such as using Gamma ray [102], bio-based inactivation methods like bio-active lipid [103], and nanomaterial [104] are examined and proposed by many scientists. However, several studies focused on the environmental parameters that have impacted on viability of the SRAS-CoV-2 such as the fixation (retention), pH, temperature, and solar radiation. For example, it was reported that pH in a wide range (pH = 3–10) has an insignificant effect on the SRAS-CoV-2 [105] while at extreme pH range (2–3 & 11–12) the virus lost its infectious just in one day [106]. Among all of the aforementioned parameters, we stepped the effect of temperature (thermal inactivation/heating) and UV into the spotlight because these two parameters.
7.1. Susceptibility of CoVs to temperature
As discussed before, in the SODIS process the two parameters of temperature and UV are crucial for an effective performance. Since the family of the coronavirus is vulnerable to temperature, it was reported that various types of coronavirus (such as HCoV-229E, SARS-CoV-1, MERS-CoV, HCoV-OC43, TGEV, MCoV, and SARS-CoV-2) on different surfaces (steel, glass, paper, cloth, latex, ceramic, wood, and cardboard) in the temperature range of 4–25 °C can survive between several hours to 28 days [107]. Furthermore, the impact of temperature on the survival of the coronavirus family in different liquids (such as Dulbecco's Modified Eagle's medium “DMEM”, Minimum Essential Medium “MEM”, hydroxyethyl piperazineethanesulfonic acid “HEPES”, phosphate-buffered saline “PBS”) including water is realized. It was reported that for the aforementioned families of the coronavirus in a temperature between 4 and 80 °C the virus can remain viable around 1 min up to 49 days respectively. The lowest temperature leads to higher survival time and vice versa [107]. These results elucidate the important fact that the family of the coronavirus is tremendously susceptible to temperature. In this regard, some researchers focused on the effect of temperature on the viability of the SARS-CoV-2. For instance, it was proved that by immersing face masks in the water at 56 °C for half an hour, the virus is eliminated and the masks can be reused [108]. Chin et al. reported that the variation of temperature highly affected viability of the SARS-CoV-2 where at 4 °C just 0.7log10 reduction was observed and the virus remained active for 14 days but at 70 °C the virus is inactivated in 5 min [105]. Another study declared that the SARS-CoV-2 virus suspended in solution in temperatures 4, 20–25, and 33–37 °C remains viable up to 14, 7, and 1–2 days respectively [106]. Recently, survival of the novel coronavirus in different solutions such as tap water, autoclave wastewater, and untreated wastewater for a varied range of temperature is realized. The results revealed that the T90 of the SARS-CoV-2 for the temperatures of 4 °C, 15 °C, 25 °C, and 37 °C for untreated wastewater, autoclaved wastewater, and tap water are varied between 8–27, 5–43, and 9–58 days respectively [109].
7.2. Effect of UV on the SARS-CoVs
Various types of UV and their wavelengths are presented in the previous sections. Effect of various types of UV on the family of coronavirus is also examined. After the SARS-CoV-1 outbreak, several studies were conducted on the effect of various types of UV on viability of the SARS-CoV-1. It was examined that UVA cannot be effective enough to eliminate the SARS-CoV-1 [110] while removing the virus by UVC was completely achieved [110]. However, in another study complete inactivation of the SARS-CoV-1by utilizing UVC was not achieved [111] which means that the results of different studies are not conclusive. Currently, numerous studies on the effect of the UV irradiance on elimination of the SARS-CoV-2 were conducted which most of them are focused on utilizing UVC. It was declared that the UVC wavelength is strong enough for eliminating the SARS-CoV-2 in different surfaces [[112], [113], [114]]. Kitagawa et al. [115] reported 88.5–99.7% diminishment in the SARS-CoV-2 concentration on different surfaces using UVC (222 nm) while Heilingloh et al. [116] reported the minimum dose of UVC for complete inactivating of the SARS-CoV-2 is 1048 mJ/cm2. Moreover, rapid inactivation of the dried SAR-CoV-2 on steel in 6–8 min can be attained by UVB irradiance with simulated natural light in the indoor experiment [117]. Still, the effect of various types of UV on contaminated solutions by SARS-CoV-2 remains unknown. Since the UVC is completely absorbed in the atmosphere and just 5% of UVB is reaching on the earth, the most abundant type of UV is UVA, but based on experiments, the UVA has an insignificant effect for deactivating the SARS-CoV-2 virus [116]. However, based on a model, the UVA may have a contribution to inactivate the virus [118]. But it should reminded that during a devastating incident such as the COVID-19, scientists and governments should avoid any announcement, speculation, and assumption in the absence of concrete scientific evidence [119]. In fact, the peak of absorbing the ultraviolet radiation in the nucleic acid of the viral is between 260 and 265 nm (the UVC range) which is placed in the optimum and effective range of germicidal wavelength [34]. Since the UV wavelength below 320 nm consider as actinic, and absorption of the UVA by the viral nucleic acid is inadequate at wavelength > 320 nm, the UVA generally has not germicidal effect [34]. The wavelength has a reverse relationship with its germicidal characteristics which means by decreasing the wavelength germicidal effect is increases and vice versa [120]. While the main effect of the UV irradiance in SODIS translates by the formation of ROSs rather than the direct effect; yet, there is no research on the effect of excessive ROSs on the SARS-CoV-2 RNA. However, we should emphasize that viruses are always introduced as one of the most resistant pathogens in the SODIS.
8. What is the risk of using SODIS during the pandemic?
As mentioned above, the ongoing pandemic contaminated water bodies in different regions of the world whether in industrialized and high-income countries or in the developing world. While presence of the SARS-CoV-2 in a high-income country like Japan [95] with high-standard of WWTPs in secondary treated wastewater is detected, the situation in the developing countries which most of them have no access to WWTPs and proper sanitation system is certainly worse. Solar disinfection is a technology that is used in developing countries rather than high-income countries and the number of people that used this method to consume drinkable water is significant. The effectiveness of the SODIS system is depended on various parameters which the most important of them are: UV, water temperature, ROSs, and type of pathogens. Generally, the SODIS method is more preferable and practical for contaminated water with bacteria rather than viruses, because among the waterborne diseases, viruses are the most resistant type compare to the others [56]. Two important parameters that are directly related to effectiveness of the SODIS performance, as well as resistance and survivability of the SARS-CoV-2, are temperature and UV. In the literature, we discuss the effect of each of these parameters on both of them. In numerous studies, researchers declared that the UVC wavelength is strong enough to eliminate the virus on different surfaces [[112], [113], [114]]. However, effect of the UVC on the SARS-CoV-2 virus in solutions (various water matrices such as contaminated water) is not realized yet and it is not clear that how the UVC wavelength is effective for inactivating the virus in different solutions.
On the other hand, the SODIS method mainly relies on the direct and indirect effect of UV irradiance while the most abundant available UV (UVA) is not adequately powerful to affect the genome directly and its effect is generally through the formation of ROS [16,21]. As we mentioned before, viruses like other pathogens can be damaged by a direct effect on the genome and indirect endogenous or exogenous effect while due to simple structure of viruses, the endogenous damage can be overlooked [59]. The main photo-inactivation of viruses on the surface of the earth is in the range of 280–315 nm of wavelengths (UVB) [59] while only 5% of the UVB is available on the earth and just a small amount of available UVB can be absorbed by genome [21]. Although the direct effect of UVA on the SARS-CoV-2 is not realized yet, but regarding numerous studies for inactivation of the novel coronavirus by UV, it can be concluded that the available UV on earth (i.e. type A: 315–400 nm) is not strong enough to damage the viral RNA. It should be mentioned that the effective wavelength for direct damage of the genome of pathogens (and highest absorption of the UV by nucleic acid) is between 250 and 270 nm which is the UVC wavelength regions [33,34,119]. Furthermore, using up-conversion materials (lanthanide elements) to turn the UVA into UVC wavelength is still stuck in laboratory stages and has many obstacles such as low quantum yield and high reactivity. In this regard, it is worthy to be mentioned that materials with high efficiency should be utilized to reach a significant level of inactivating pathogens in water to avoid the mechanism of repair. The indirect effect of UV by producing free radicals is also questionable. Powerful oxidants such as hydroxyl radicals are short-lived and tremendously reactive and subsequently, it is not effective in complex water matrices which contains natural organic matters (such as wastewaters) [25,26]. Furthermore, effect of the singlet oxygen radicals which is more effective in complex water matrices as well as on enveloped viruses remains in question because the photosensitizers should separate from the water before it consumes by individuals which leads to decrease the effectiveness of SODIS system [33]. As mentioned before, the SARS-CoV-2 virus is highly vulnerable to an increase in temperature and it can be eliminated because of the destruction of its lipid and protein. Thus, thermal inactivation is proposed as a powerful method to eliminate the virus. It should be mentioned that viability of the virus has an inverse relation with temperature. As it discussed before the T90 of the SARS-CoV-2 for various temperatures 4 °C, 15 °C, 25 °C, and 37 °C in different wastewater samples is varied between 5 and 58 days [109] (Fig. 4 ) whereas in the SODIS method; under real conditions based on experiments, temperature of water is below 40 °C and even in ideal conditions (i.e., summer conditions when solar intensity and ambient temperature stands at the highest point) the water temperature may not reach 40 °C [35,40,42]. The low temperature of water in the SODIS has two issues. First, the synergistic effect of temperature with UV can be obtained only and if only the water temperature is higher than 45 °C [38] while for temperature >45 °C ideal conditions are required (i.e., hot summers). Secondly, at lower temperatures, the SARS-CoV-2 virus is more resistant and it can remain viable where at 37 °C the T90 for different types of wastewater samples and tap water contaminated by the novel coronavirus is around 5–8 days [109]. Moreover, in other seasons (except summer) the environmental condition is not suitable for the SODIS due to the lower solar intensity and ambient temperature of water is low. Subsequently, the viral would be remaining longer days viable in water (up to 58 days). To overcome the problem of UV and low temperature in SODIS, up-conversion materials and nanoparticles for turning the UVA to UVC and increasing the water temperature by trapping light and taking the advantage of heat localization [36,121] are proposed. But neither of these solutions approaches to practical application that can be widely used by poor communities because of obstacles (specific characteristics of materials, cost of materials, advanced methods for preparing the apparatus) in mass production, thus these methods remain in laboratory scales.
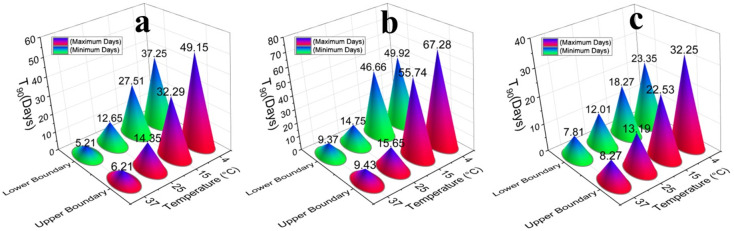
Fig. 4.
T90 of the SARS-CoV-2 for various wastewaters and tap water samples with respect to water temperature. By increasing the water temperature the T90 is decreased and vice versa. The upper and lower boundaries represent the maximum and minimum T90 of the samples in different temperature considering standard deviation a) autoclaved wastewater (standard deviations for temperature 4 °C, 15 °C, 25 °C, and 37 °C are ±5.95, ±2.39, ±0.85, and ±0.5 respectively), b) tap water (standard deviations for temperature 4 °C, 15 °C, 25 °C, and 37 °C are ±8.68, ±4.54, ±0.45, and ±0.03 respectively), c) untreated wastewater (standard deviations for temperature 4 °C, 15 °C, 25 °C, and 37 °C are ±4.45, ±2.13, ±0.59, and ±0.23 respectively).
9. Summaries, concluding remarks, and knowledge gaps
In the present study, reliability of solar water disinfection during the COVID-19 pandemic is discussed. The following summaries and remarks can be concluded.
-
•
A huge amount of water bodies via different routes are contaminated due to the ongoing pandemic.
-
•
Presence of the SARS-CoV-2 in different water matrices including untreated wastewater, treated wastewater, rivers, freshwater environment, and groundwater is detected all around the globe.
-
•
Presence of the virus (at higher concentration) at water bodies in developing countries due to absence or ineffective sanitation system and WWTPs is higher than industrialized and high-income countries
-
•
The novel coronavirus can be viable within several days (5–58 days) in different temperatures and different solutions.
-
•
Solar radiation, temperature, physicochemical characteristics of water, features of virus, type and length of the genome, composition of adjacent pyrimidine dimers or guanine content are the factors that affect sunlight-mediated disinfection of viruses.
-
•
UV irradiance and temperature are the two important effective parameters that SODIS and novel coronavirus have in common.
-
•
Typically, the SODIS method is more practical for contaminated water by bacteria than viruses
-
•
Most of studies in SODIS system focused on eliminating various type of the bacteria rather than other pathogens
-
•
The most abundant available type of UV on the surface of earth (UVA) is not strong enough to eliminate viruses.
-
•
The effectiveness of UVA in the SODIS system is through formation of ROSs
-
•
The effective UV wavelength for viral eliminating is in the range of 100–280 nm which consider as the UVC
-
•
To achieve the synergistic effect of thermal-optic in viral deactivation the temperature of water should be higher than 40 °C.
-
•
Performance of SODIS in cold seasons is tremendously decreases because of the lower ambient temperature and solar (i.e. available UV) intensity
-
•
The SARS-CoV-2 virus in temperature of 37 °C remains viable in autoclave wastewater, untreated wastewater, and tap water around 5, 8, and 9 days respectively.
-
•
In SODIS system reaching to higher temperature (>40 °C) of water can be realize only in summer and hot climate conditions.
-
•
Rate of reduction for the novel coronavirus in different medium (surfaces, solutions, etc.) has an inverse relation with temperature.
-
•
The critical temperature for killing the SARS-Cov-2 is >56 °C (its eliminated in less than 30 min) while most of SODIS systems worked at temperature lower than 45 °C.
Furthermore, the following are recommended as the post-pandemic studies
-
•
Realizing the effectiveness of SODIS system with contaminated water by the novel coronavirus in different seasons, especially, cold seasons.
-
•
Determining the important parameters on the performance of SODIS system (fed by SARS-CoV-2 contaminated water) such as variable UV intensity, water temperature, turbidity, time of exposure.
-
•
How the available UVA can affected the SRAS-CoV-2 viability in contaminated water.
-
•
How concentration of the virus during the SODIS process is changed when one parameter is variable and the other parameters are constant.
-
•
Determining the effect of originated free radicals during the SODIS process (i.e., hydroxyl and dioxygen) on viability of the SARS-CoV-2 in water.
-
•
How different physical modifications for SODIS containers such as increasing the rate of absorption in containers can affected viability of the virus.
-
•
How utilizing advance materials such as lanthanide elements during the SODIS process would be practical in eliminating the SARS-CoV-2 in contaminated water.
-
•
Determining the proper dosage of UVB for deactivation of the SARS-CoV-2 under laboratory and natural environmental conditions.
10. Conclusion
The ongoing pandemic forced too many stresses on all communities from different aspects. These pressures on people in developing countries and poor communities are more than in high-income countries because of the lower standard of living. Also, increasing the number of infections imposes many environmental barriers to water bodies and aquatic environments by contaminating them with the virus. Using SODIS system regarding the potential ineffectiveness for eliminating pathogens (in this case the SARS-CoV-2) during a situation like the COVID-19 pandemic (or any similar situation) that water bodies are contaminated could have many risks to open a new route for infection and transmission. However, the infectious by water matrices remains in question due to lack of valid data and many uncertainties that involve, but researchers have speculations about the infection of individuals due to contaminated water [7,92]. Once again, it should be reminded that when the rate of fatalities are more than it was anticipated before [122], any possibility about the SARS-CoV-2 transmission must be consider by experts in related fields, that means new ideas and theories which are based on concrete pieces of evidences should be embraced by scientific community [119].
CRediT authorship contribution statement
Seyed Masoud Parsa: Conceptualization, Methodology, Investigation, Visualization, Writing - Original Draft, Resources, Supervision, Project Administration. Saba Momeni: Developed the DNA mechanism section, Data Curation, Writing - Original Draft, Visualization, Investigation, Writing - Review & Editing. Ahmad Reza Hemmat: Writing - Review & Editing. Masoud Afrand: Writing - Review & Editing, Resources.
Declaration of competing interest
None.
References
- 1.Teymourian T., Teymoorian T., Kowsari E., Ramakrishna S. Challenges, strategies, and recommendations for the huge surge in plastic and medical waste during the global COVID-19 pandemic with circular economy approach. Mater. Circ. Econ. 2021;3 doi: 10.1007/s42824-021-00020-8. [DOI] [Google Scholar]
- 2.Zeng G., Wang L., Zhang Z. Prejudice and xenophobia in COVID-19 research manuscripts. Nat. Hum. Behav. 2020;4:879. doi: 10.1038/s41562-020-00948-y. [DOI] [PubMed] [Google Scholar]
- 3.Chavarria-Miró G., Anfruns-Estrada E., Guix S., Paraira M., Galofré B., Sáanchez G., Pintó R., Bosch A. Sentinel surveillance of SARS-CoV-2 in wastewater anticipates the occurrence of COVID-19 cases. MedRxiv. 2020 doi: 10.1101/2020.06.13.20129627. 2020.06.13.20129627. [DOI] [Google Scholar]
- 4.Fongaro G., Stoco P.H., Souza D.S.M., Grisard E.C., Magri M.E., Rogovski P., Schorner M.A., Barazzetti F.H., Christoff A.P., Oliveira L.F.V. de, Bazzo M.L., Wagner G., Hernandez M., Rodriguez-Lazaro D. SARS-CoV-2 in human sewage in Santa Catalina, Brazil, November 2019. MedRxiv. 2020 doi: 10.1101/2020.06.26.20140731. 2020.06.26.20140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilal M., Nazir M.S., Rasheed T., Parra-Saldivar R., Iqbal H.M.N. Water matrices as potential source of SARS-CoV-2 transmission – an overview from environmental perspective. Case Stud. Chem. Environ. Eng. 2020 doi: 10.1016/j.cscee.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsa S.M. Reliability of thermal desalination (solar stills) for water/wastewater treatment in light of COVID-19 (novel coronavirus “SARS-CoV-2”) pandemic: what should consider? Desalination. 2021 doi: 10.1016/j.desal.2021.115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mekonnen M.M., Hoekstra A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016;2:1–7. doi: 10.1126/sciadv.1500323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsa S.M., Rahbar A., Javadi D.Y., Koleini M.H., Afrand M., Amidpour M. Energy-matrices, exergy, economic, environmental, exergoeconomic, enviroeconomic, and heat transfer (6E/HT) analysis of two passive/active solar still water desalination nearly 4000 m: altitude concept. J. Clean. Prod. 2020;261 doi: 10.1016/j.jclepro.2020.121243. [DOI] [Google Scholar]
- 11.Brazil May Be the Owner of 20% of the World's Water Supply But It Is Still Very Thirsty. https://www.worldbank.org/en/news/feature/2016/07/27/how-brazil-managing-water-resources-new-report-scd n.d.
- 12.Parsa S.M., Rahbar A., Koleini M.H., Javadi Y. Davoud, Afrand M., Rostami S., Amidpour M. First approach on nanofluid-based solar still in high altitude for water desalination and solar water disinfection (SODIS) Desalination. 2020;491 doi: 10.1016/j.desal.2020.114592. [DOI] [Google Scholar]
- 13.Parsa S.M., Javadi D.Y., Rahbar A., Majidniya M., Salimi M., Amidpour Y., Amidpour M. Experimental investigation at a summit above 13,000 ft on active solar still water purification powered by photovoltaic: a comparative study. Desalination. 2020;476 doi: 10.1016/j.desal.2019.114146. [DOI] [Google Scholar]
- 14.Parsa S.M., Javadi D.Y., Rahbar A., Majidniya M., Aberoumand S., Amidpour Y., Amidpour M. Experimental assessment on passive solar distillation system on Mount Tochal at the height of 3964 m: study at high altitude. Desalination. 2019;466:77–88. doi: 10.1016/j.desal.2019.05.010. [DOI] [Google Scholar]
- 15.Parsa S.M., Rahbar A., Koleini M.H., Aberoumand S., Afrand M., Amidpour M. A renewable energy-driven thermoelectric-utilized solar still with external condenser loaded by silver/nanofluid for simultaneously water disinfection and desalination. Desalination. 2020 doi: 10.1016/j.desal.2020.114354. [DOI] [Google Scholar]
- 16.McGuigan K.G., Conroy R.M., Mosler H.J., Preez M. du, Ubomba-Jaswa E., Fernandez-Ibañez P. Solar water disinfection (SODIS): a review from bench-top to roof-top. J. Hazard. Mater. 2012;235–236:29–46. doi: 10.1016/j.jhazmat.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 17.Marugán J., Giannakis S., McGuigan K.G., Polo-López I. 2020. Solar Disinfection as a Water Treatment Technology; pp. 1–16. (Clean Water Sanit.). [DOI] [Google Scholar]
- 18.Castro-Alférez M., Polo-López M.I., Marugán J., Fernández-Ibáñez P. Mechanistic model of the Escherichia coli inactivation by solar disinfection based on the photo-generation of internal ROS and the photo-inactivation of enzymes: CAT and SOD. Chem. Eng. J. 2017;318:214–223. doi: 10.1016/j.cej.2016.06.093. [DOI] [Google Scholar]
- 19.Das K., Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014;2:1–13. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- 20.Castro-Alférez M., Polo-López M.I., Fernández-Ibáñez P. Intracellular mechanisms of solar water disinfection. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep38145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha R.P., Häder D.P. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 22.Al-Gheethi A.A.S., Noman E.A., Mohamed R.M.S. Radin, Talip B.A., Kassim A.H. Mohd, Ismail N. Springer International Publishing; 2019. Disinfection Technologies for Household Greywater. [DOI] [Google Scholar]
- 23.Yin H., Xu L., Porter N.A. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 24.Cho M., Yoon J. Measurement of OH radical CT for inactivating Cryptosporidium parvum using photo/ferrioxalate and photo/TiO2 systems. J. Appl. Microbiol. 2008;104:759–766. doi: 10.1111/j.1365-2672.2007.03682.x. [DOI] [PubMed] [Google Scholar]
- 25.Hodges B.C., Cates E.L., Kim J.H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018;13:642–650. doi: 10.1038/s41565-018-0216-x. [DOI] [PubMed] [Google Scholar]
- 26.Brame J., Long M., Li Q., Alvarez P. Trading oxidation power for efficiency: differential inhibition of photo-generated hydroxyl radicals versus singlet oxygen. Water Res. 2014;60:259–266. doi: 10.1016/j.watres.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Latch D.E., McNeill K. Microheterogeneity of singlet oxygen distributions in irradiated humic acid solutions. Science (80-.) 2006;311:1743–1747. doi: 10.1126/science.1121636. [DOI] [PubMed] [Google Scholar]
- 28.Kim H., Kim W., MacKeyev Y., Lee G.S., Kim H.J., Tachikawa T., Hong S., Lee S., Kim J., Wilson L.J., Majima T., Alvarez P.J.J., Choi W., Lee J. Selective oxidative degradation of organic pollutants by singlet oxygen-mediated photosensitization: tin porphyrin versus C60 aminofullerene systems. Environ. Sci. Technol. 2012;46:9606–9613. doi: 10.1021/es301775k. [DOI] [PubMed] [Google Scholar]
- 29.Mi. Hamblin G. Jori. 2011. PDT Inactivation of Microbial Pathogens - Medical and Environmental Applications - Benhamou et al Publication.pdf; p. 36. [Google Scholar]
- 30.Vigant F., Santos N.C., Lee B. Broad-spectrum antivirals against viral fusion. Nat. Rev. Microbiol. 2015;13:426–437. doi: 10.1038/nrmicro3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J., MacKeyev Y., Cho M., Wilson L.J., Kim J.H., Alvarez P.J.J. C60 aminofullerene immobilized on silica as a visible-light-activated photocatalyst. Environ. Sci. Technol. 2010;44:9488–9495. doi: 10.1021/es1028475. [DOI] [PubMed] [Google Scholar]
- 32.Moor K.J., Kim J.H. Simple synthetic method toward solid supported C60 visible light-activated photocatalysts. Environ. Sci. Technol. 2014;48:2785–2791. doi: 10.1021/es405283w. [DOI] [PubMed] [Google Scholar]
- 33.Chu C., Ryberg E.C., Loeb S.K., Suh M.J., Kim J.H. Water disinfection in rural areas demands unconventional solar technologies. Acc. Chem. Res. 2019;52:1187–1195. doi: 10.1021/acs.accounts.8b00578. [DOI] [PubMed] [Google Scholar]
- 34.Kowalski W. 2009. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection. [DOI] [Google Scholar]
- 35.Rincón A.G., Pulgarin C. Field solar E. coli inactivation in the absence and presence of TiO2: is UV solar dose an appropriate parameter for standardization of water solar disinfection? Sol. Energy. 2004;77:635–648. doi: 10.1016/j.solener.2004.08.002. [DOI] [Google Scholar]
- 36.Zhang W., Yang S., Li J., Gao W., Deng Y., Dong W., Zhao C., Lu G. Environmental visible-to-ultraviolet upconvertion: energy transfer, material matrix, and synthesis strategies. Appl. Catal. B Environ. 2017;206:89–103. doi: 10.1016/j.apcatb.2017.01.023. [DOI] [Google Scholar]
- 37.Vivar M., Pichel N., Fuentes M. Solar disinfection of natural river water with low microbiological content (10–103 CFU/100 ml) and evaluation of the thermal contribution to water purification. Sol. Energy. 2017;141:1–10. doi: 10.1016/j.solener.2016.11.019. [DOI] [Google Scholar]
- 38.Mcguigan K.G., Joyce T.M., Conroy R.M., Gillespie J.B. Solar disinfection of drinking water contained in transparent plastic bottles: characterizing the bacterial inactivation process. J. Appl. Microbiol. 1998:1138–1148. doi: 10.1046/j.1365-2672.1998.00455.x. [DOI] [PubMed] [Google Scholar]
- 39.Theitler D.J., Nasser A., Gerchman Y., Kribus A., Mamane H. Synergistic effect of heat and solar UV on DNA damage and water disinfection of E. coli and bacteriophage MS2. J. Water Health. 2012;10:605–618. doi: 10.2166/wh.2012.072. [DOI] [PubMed] [Google Scholar]
- 40.Sichel C., Blanco J., Malato S., Fernández-Ibáñez P. Effects of experimental conditions on E. coli survival during solar photocatalytic water disinfection. J. Photochem. Photobiol. A Chem. 2007;189:239–246. doi: 10.1016/j.jphotochem.2007.02.004. [DOI] [Google Scholar]
- 41.Gomez-Couso H., Fontan M., Sichel C., Ferna P., Go H. Efficacy of the solar water disinfection method in turbid waters experimentally contaminated with Cryptosporidium parvum oocysts under real field conditions. Trop. Med. Int. Heal. 2009;14:620–627. doi: 10.1111/j.1365-3156.2009.02281.x. [DOI] [PubMed] [Google Scholar]
- 42.Sichel C., Tello J., Cara M. de, Fernández-Ibáñez P. Effect of UV solar intensity and dose on the photocatalytic disinfection of bacteria and fungi. Catal. Today. 2007;129:152–160. doi: 10.1016/j.cattod.2007.06.061. [DOI] [Google Scholar]
- 43.Saitoh T.S., EL-Ghetany H.H. 2000. Exposure Time as a Disinfecting Index in a Solar Water Disinfecting System; pp. 1162–1166. [Google Scholar]
- 44.Davies-Colley R.J., Craggs R.J., Park J., Sukias J.P.S., Nagels J.W., Stott R. Virus removal in a pilot-scale “advanced” pond system as indicated by somatic and F-RNA bacteriophages. Water Sci. Technol. 2005;51:107–110. doi: 10.2166/wst.2005.0440. [DOI] [PubMed] [Google Scholar]
- 45.Wegelin M., Canonica S., Mechsner K., Fleischmann F., Pesaro F., Metzler A. Solar water disinfection: scope of the process and analysis of radiation experiments. J. Water SRT–Aqua. 1994:154–169. [Google Scholar]
- 46.Boyle M., Sichel C., Ferna P. Bactericidal effect of solar water disinfection under real sunlight conditions. Appl. Environ. Microbiol. 2008;74:2997–3001. doi: 10.1128/AEM.02415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kehoe S.C., Barer M.R., Devlin L.O., Mcguigan K.G. 2004. Batch Process Solar Disinfection Is an Efficient Means of Disinfecting Drinking Water Contaminated With Shigella dysenteriae Type I; pp. 410–414. [DOI] [PubMed] [Google Scholar]
- 48.Lonnen J., Kilvington S., Kehoe S.C., Al-Touati F., McGuigan K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 2005;39:877–883. doi: 10.1016/j.watres.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 49.Heaselgrave W., Patel N., Kilvington S., Kehoe S.C., Mcguigan K.G. Solar disinfection of poliovirus and Acanthamoeba polyphaga cysts in water – a laboratory study using simulated sunlight. Lett. Appl. Microbiol. 2006;43:125–130. doi: 10.1111/j.1472-765X.2006.01940.x. [DOI] [PubMed] [Google Scholar]
- 50.Heaselgrave W., Kilvington S. The efficacy of simulated solar disinfection (SODIS) against Ascaris, Giardia, Acanthamoeba, Naegleria, Entamoeba and Cryptosporidium. Acta Trop. 2011;119:138–143. doi: 10.1016/j.actatropica.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Polo D., García-fernández I., Fernández-ibáñez P., Romalde J.L. 2015. Solar Water Disinfection (SODIS): Impact on Hepatitis A Virus and on a Human Norovirus Surrogate Under Natural Solar Conditions; pp. 41–49. [DOI] [PubMed] [Google Scholar]
- 52.Angeler D.G., Fried-petersen H., Allen C.R., Garmestani A., Chuang W., Donovan V.M., Eason T., Roberts C.P., Wonkka C.L., Survey G., Fish N.C., Management R., U.S.E.P. Agency, Hall K. Sunlight-mediated inactivation of health-relevant microorganisms in water: a review of mechanisms and modeling approaches. Adv. Ecol. Res. 2019;60:1–24. doi: 10.1039/c8em00047f.Sunlight-mediated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oguma K., Katayama H., Mitani H., Morita S., Hirata T., Ohgaki S. Determination of pyrimidine dimers in Escherichia coli and Cryptosporidium parvum during UV light inactivation, photoreactivation, and dark repair. Appl. Environ. Microbiol. 2001;67:4630–4637. doi: 10.1128/AEM.67.10.4630-4637.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kavakli I.H., Baris I., Tardu M., Gül Ş., Öner H., Çal S., Bulut S., Yarparvar D., Berkel Ç., Ustaoğlu P., Aydın C. The photolyase/cryptochrome family of proteins as DNA repair enzymes and transcriptional repressors. Photochem. Photobiol. 2017;93:93–103. doi: 10.1111/php.12669. [DOI] [PubMed] [Google Scholar]
- 55.Maclean M., Murdoch L.E., Lani M.N., MacGregor S.J., Anderson J.G., Woolsey G.A. Photoinactivation and photoreactivation responses by bacterial pathogens after exposure to pulsed UV-light. Proc. 2008 IEEE Int. Power Modul. High Volt. Conf. PMHVC. 2008:326–329. doi: 10.1109/IPMC.2008.4743649. [DOI] [Google Scholar]
- 56.Nwankwo E.J., Agunwamba J.C., Nnaji C.C. Effect of radiation intensity, water temperature and support-base materials on the inactivation efficiency of solar water disinfection (SODIS) Water Resour. Manag. 2019;33:4539–4551. doi: 10.1007/s11269-019-02407-4. [DOI] [Google Scholar]
- 57.Zhang C., Li Y., Shuai D., Shen Y., Wang D. Progress and challenges in photocatalytic disinfection of waterborne viruses: a review to fill current knowledge gaps. Chem. Eng. J. 2019;355:399–415. doi: 10.1016/j.cej.2018.08.158. [DOI] [Google Scholar]
- 58.Goodsell D.S. The molecular perspective: ultraviolet light and pyrimidine dimers. Oncologist. 2001:348–349. doi: 10.1634/stemcells.19-4-348. [DOI] [PubMed] [Google Scholar]
- 59.García-Gil Á., Martínez A., Polo-López M.I., Marugán J. Kinetic modeling of the synergistic thermal and spectral actions on the inactivation of viruses in water by sunlight. Water Res. 2020:183. doi: 10.1016/j.watres.2020.116074. [DOI] [PubMed] [Google Scholar]
- 60.Eisenstark A. Mutagenic and lethal effects of near-ultraviolet radiation (290–400 nm) on bacteria and phage. Environ. Mol. Mutagen. 1987;10:317–337. doi: 10.1002/em.2850100311. [DOI] [PubMed] [Google Scholar]
- 61.Reed R.H., Mani S.K., Meyer V. Solar photo-oxidative disinfection of drinking water: preliminary field observations. Lett. Appl. Microbiol. 2000;30:432–436. doi: 10.1046/j.1472-765x.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- 62.Mattle M.J., Vione D., Kohn T. Conceptual model and experimental framework to determine the contributions of direct and indirect photoreactions to the solar disinfection of MS2, phiX174, and adenovirus. Environ. Sci. Technol. 2015;49:334–342. doi: 10.1021/es504764u. [DOI] [PubMed] [Google Scholar]
- 63.Lytle C.D., Sagripanti J. Predicted inactivation of viruses of relevance to biodefense by solar radiation. 2005;79:14244–14252. doi: 10.1128/JVI.79.22.14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rainbow A.J., Mak S. 1973. DNA Damage and Biological Function of Human Adenovirus After US.-Irradiation. [DOI] [PubMed] [Google Scholar]
- 65.Johns H.E., Pearso M. Suppression of hydrate and dimer formation in traviolet-irradiated poly (A + U) relative to poly. J. Mol. Biol. 1966:215–229. doi: 10.1016/0022-2836(66)90061-1. [DOI] [PubMed] [Google Scholar]
- 66.Swenson P.A., Setlo R.B. Kinetics of dimer formation and photohydration in ultraviolet-irradiated polyuridylic acid. Photochem. Photohiol. 1963;2:419–434. [Google Scholar]
- 67.Mitchell L., Clarkson J.M. Induction of photoproducts in synthetic polynucleotides by far and near ultraviolet radiation. Photochem. Photohiol. 1984;40:735–741. doi: 10.1111/j.1751-1097.1984.tb04645.x. [DOI] [PubMed] [Google Scholar]
- 68.Alotaibi M.A., Heaselgrave W. Solar disinfection of water for inactivation of enteric viruses and its enhancement by riboflavin. Food Environ. Virol. 2011;3:70–73. doi: 10.1007/s12560-011-9058-5. [DOI] [Google Scholar]
- 69.Baert L., Wobus C.E., Coillie E. Van, Thackray L.B., Debevere J., Uyttendaele M. Detection of murine norovirus 1 by using plaque assay, transfection assay, and real-time reverse transcription-PCR before and after heat exposure. Appl. Environ. Microbiol. 2008;74:543–546. doi: 10.1128/AEM.01039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hewitt J., Rivera-Aban M., Greening G.E. Evaluation of murine norovirus as a surrogate for human norovirus and hepatitis A virus in heat inactivation studies. J. Appl. Microbiol. 2009;107:65–71. doi: 10.1111/j.1365-2672.2009.04179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nuanualsuwan S., Cliver D.O. Capsid functions of inactivated human picornaviruses and feline calicivirus. Appl. Environ. Microbiol. 2003;69:350–357. doi: 10.1128/AEM.69.1.350-357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Best J. Anthropogenic stresses on the world’ s big rivers. Nat. Geosci. 2019;12 doi: 10.1038/s41561-018-0262-x. [DOI] [Google Scholar]
- 73.Dan P., Thanh B., Truong D. Case studies of groundwater pollution in Southeast Vietnam. Int. Rev. Environ. Strateg. 2006;6:361–371. http://connection.ebscohost.com/c/articles/21712934/case-studies-groundwater-pollution-southeast-vietnam [Google Scholar]
- 74.Henry R.K., Yongsheng Z., Jun D. Municipal solid waste management challenges in developing countries - Kenyan case study. Waste Manag. 2006;26:92–100. doi: 10.1016/j.wasman.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Lekwot V.E., Nunyi B.V., Ifeanyi E., I C., Adamu B. Public health implication of improper hospital waste disposal in Zonkwa district of Zangon-kataf local government area, Kaduna state. J. Res. Environ. Sci. Toxicol. 2012;1:23–28. http://www.interesjournals.org/JREST [Google Scholar]
- 76.Cofie O.O., Kranjac-Berisavljevic G., Drechsel P. The use of human waste for peri-urban agriculture in Northern Ghana. Renew. Agric. Food Syst. 2005;20:73–80. doi: 10.1079/raf200491. [DOI] [Google Scholar]
- 77.Kranjac-Berisavljevic G., Cofie O. Faecal sludge application for agriculture in Tamale. Urban Agric. Mag. 2003:31–33. [Google Scholar]
- 78.Okoh A.I., Sibanda T., Gusha S.S. Inadequately treated wastewater as a source of human enteric viruses in the environment. Int. J. Environ. Res. Public Health. 2010;7:2620–2637. doi: 10.3390/ijerph7062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80:6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodriguez D.J., Serrano H. Alexander, Delgado A., Nolasco D., Saltiel G. 2019. From Waste to Resource - Shifting Paradigms for Smarter Wastewater Interventions in Latin America and the Caribbean. [DOI] [Google Scholar]
- 81.Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Downs T.J., Cifuentes-García E., Suffet I.M. Risk screening for exposure to groundwater pollution in a wastewater irrigation district of the Mexico City region. Environ. Health Perspect. 1999;107:553–561. doi: 10.1289/ehp.99107553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Srivastava A., Srivastava S. Assessment of physico-chemical properties and sewage pollution indicator bacteria in surface water of River Gomti in Uttar Pradesh. Int. J. Environ. Sci. 2011;2:325–336. [Google Scholar]
- 84.Hamner S., Tripathi A., Mishra R.K., Bouskill N., Broadaway S.C., Pyle B.H., Ford T.E. The role of water use patterns and sewage pollution in incidence of water-borne/enteric diseases along the Ganges River in Varanasi, India. Int. J. Environ. Health Res. 2006;16:113–132. doi: 10.1080/09603120500538226. [DOI] [PubMed] [Google Scholar]
- 85.Rizzo A., Tondera K., Pálfy T.G., Dittmer U., Meyer D., Schreiber C., Zacharias N., Ruppelt J.P., Esser D., Molle P., Troesch S., Masi F. Constructed wetlands for combined sewer overflow treatment: a state-of-the-art review. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138618. [DOI] [PubMed] [Google Scholar]
- 86.Transboundary River Basins: Status and Trends (UNEP-DHI, UNEP, TWAP, 2016) 2016. http://gefwap.org/publications/river-basins-technical-report
- 87.Kumar M., Alamin M., Kuroda K., Dhangar K., Hata A., Yamaguchi H., Honda R. Potential discharge, attenuation and exposure risk of SARS-CoV-2 in natural water bodies receiving treated wastewater. Npj Clean Water. 2021;4:1–11. doi: 10.1038/s41545-021-00098-2. [DOI] [Google Scholar]
- 88.Buonerba A., Corpuz M.V.A., Ballesteros F., Choo K.H., Hasan S.W., Korshin G.V., Belgiorno V., Barceló D., Naddeo V. Coronavirus in water media: analysis, fate, disinfection and epidemiological applications. J. Hazard. Mater. 2021;415 doi: 10.1016/j.jhazmat.2021.125580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shutler J.D., Zaraska K., Holding T., Machnik M., Uppuluri K., Ashton I.G.C., Migdał Ł., Dahiya R.S. Rapid assessment of SARS-CoV-2 transmission risk for fecally contaminated river water. ACS ES&T Water. 2021;1:949–957. doi: 10.1021/acsestwater.0c00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huo C., Dar A. Ahmed, Nawaz A., Hameed J., albashar G., Pan B., Wang C. Groundwater contamination with the threat of COVID-19: insights into CSR theory of Carroll’s pyramid. J. King Saud Univ. Sci. 2021;33 doi: 10.1016/j.jksus.2020.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahlknecht J., Alonso-Padilla D., Ramos E., Reyes L.M., Álvarez M.M. The presence of SARS-CoV-2 RNA in different freshwater environments in urban settings determined by RT-qPCR: implications for water safety. Sci. Total Environ. 2021;784 doi: 10.1016/j.scitotenv.2021.147183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bogler A., Packman A., Furman A., Gross A., Kushmaro A., Ronen A., Dagot C., Hill C., Vaizel-Ohayon D., Morgenroth E., Bertuzzo E., Wells G., Kiperwas H.R., Horn H., Negev I., Zucker I., Bar-Or I., Moran-Gilad J., Balcazar J.L., Bibby K., Elimelech M., Weisbrod N., Nir O., Sued O., Gillor O., Alvarez P.J., Crameri S., Arnon S., Walker S., Yaron S., Nguyen T.H., Berchenko Y., Hu Y., Ronen Z., Bar-Zeev E. Rethinking wastewater risks and monitoring in light of the COVID-19 pandemic. Nat. Sustain. 2020 doi: 10.1038/s41893-020-00605-2. [DOI] [Google Scholar]
- 93.Mohapatra S., Menon N.G., Mohapatra G., Pisharody L., Pattnaik A., Menon N.G., Bhukya P.L., Srivastava M., Singh M., Barman M.K., Gin K.Y.H., Mukherji S. The novel SARS-CoV-2 pandemic: possible environmental transmission, detection, persistence and fate during wastewater and water treatment. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.142746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J.L., Liang Z., Peng Y., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosa G. La, Mancini P., Ferraro G. Bonanno, Veneri C., Iaconelli M., Bonadonna L., Lucentini L., Suffredini E. SARS-CoV-2 has been circulating in northern Italy since December 2019: evidence from environmental monitoring. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Westhaus S., Weber F.A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T., Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany – suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wurtzer S., Marechal V., Jm M., Moulin L., Université S., Metis U.M.R., Atelier Z. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases Eau de Paris, R & D Laboratory, DRDQE 11 Avenue Jean Jaurès 94200 Ivry/Seine, France. Corresponding authors * laurent.moulin@eaudeparis. MedRxiv. 2020:10–13. [Google Scholar]
- 99.Bar-on Y.M., Flamholz A.V.I., Phillips R.O.B., Milo R.O.N. SARS-CoV-2 (COVID-19) by the numbers. 2020;2:1–15. doi: 10.7554/eLife.57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Castaño N., Cordts S., Jalil M.K., Zhang K., Koppaka S., Paul R., Tang S.K.Y. 2020. Fomite Transmission and Disinfection Strategies for SARS-CoV-2 and Related Viruses; pp. 1–40. [Google Scholar]
- 102.Leung A., Tran K., Audet J., Lavineway S., Bastien N., Krishnan J. Vol. 25. 2020. In Vitro Inactivation of SARS-CoV-2 Using Gamma Radiation; pp. 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Das U.N. Can bioactive lipids inactivate coronavirus (COVID-19)? Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sportelli M.C., Izzi M., Kukushkina E.A., Hossain S.I., Picca R.A., Ditaranto N., Cio N. Vol. 19. 2020. Can Nanotechnology and Materials Science Help the Fight against SARS-CoV-2? pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1 doi: 10.1016/s2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chan K.H., Sridhar S., Zhang R.R., Chu H., Fung A.Y.F., Chan G., Chan J.F.W., To K.K.W., Hung I.F.N., Cheng V.C.C., Yuen K.Y. Factors affecting stability and infectivity of SARS-CoV-2. J. Hosp. Infect. 2020;106:226–231. doi: 10.1016/j.jhin.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aboubakr H.A. 2020. Stability of SARS-CoV-2 and Other Coronaviruses in the Environment and on Common Touch Surfaces and the Influence of Climatic Conditions: A Review; pp. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang D., Sun B.C., Wang J.X., Zhou Y.Y., Chen Z.W., Fang Y., Yue W.H., Liu S.M., Liu K.Y., Zeng X.F., Chu G.W., Chen J.F. Can masks be reused after hot water decontamination during the COVID-19 pandemic? Engineering. 2020:0–6. doi: 10.1016/j.eng.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P., Simpson S.L., Sirikanchana K., Symonds E.M., Verhagen R., Vasan S.S., Kitajima M., Bivins A. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Darnell M.E.R., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212:119–123. doi: 10.1159/000089211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hebling M., Vatter P. Ultraviolet irradiation doses for coronavirus inactivation – review and analysis of coronavirus photoinactivation studies Ultraviolette Bestrahlungsdosen für die Inaktivierung von Coronaviren. GMS Hyg. Infect. Control. 2020;15:1–8. doi: 10.3205/dgkh000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raeiszadeh M., Adeli B. 2020. A Critical Review on Ultraviolet Disinfection Systems Against COVID-19 Outbreak: Applicability, Validation, and Safety Considerations. [DOI] [PubMed] [Google Scholar]
- 114.Hadi J., Dunowska M., Wu S., Brightwell G. Vol. 2020. 2020. Control Measures for SARS-CoV-2: A Review on Light-based Inactivation of Single-stranded RNA Viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kitagawa H., Nomura T., Nazmul T., Omori K., Shigemoto N., Sakaguchi T., Ohge H. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control. 2020;000:17–19. doi: 10.1016/j.ajic.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heilingloh C.S., Aufderhorst U.W., Schipper L., Dittmer U., Witzke O., Yang D., Zheng X., Sutter K., Trilling M., Alt M., Steinmann E., Krawczyk A. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control. 2020;48:1273–1275. doi: 10.1016/j.ajic.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ratnesar-shumate S., Williams G., Green B., Krause M., Holland B., Wood S., Bohannon J., Boydston J., Freeburger D., Hooper I., Beck K., Yeager J., Altamura L.A., Biryukov J., Yolitz J., Schuit M., Wahl V., Hevey M., Dabisch P. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J. Infect. Dis. 2020;222 doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schuit M., Ratnesar-shumate S., Yolitz J., Williams G., Weaver W., Green B., Miller D., Krause M., Beck K., Wood S., Holland B., Bohannon J., Freeburger D., Hooper I., Biryukov J., Altamura L.A., Wahl V., Hevey M., Dabisch P. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J. Infect. Dis. 2020;222 doi: 10.1093/infdis/jiaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seyer A., Sanlidag T. Solar ultraviolet radiation sensitivity of SARS-CoV-2. Lancet Microbe. 2020;1:e8–e9. doi: 10.1016/S2666-5247(20)30013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pozo-antonio J.S., Sanmartín P. Exposure to artificial daylight or UV irradiation (A, B or C) prior to chemical cleaning: an effective combination for removing phototrophs from granite. Biofouling. 2018;0:1–19. doi: 10.1080/08927014.2018.1512103. [DOI] [PubMed] [Google Scholar]
- 121.Hogan N.J., Urban A.S., Ayala-Orozco C., Pimpinelli A., Nordlander P., Halas N.J. Nanoparticles heat through light localization. Nano Lett. 2014;14:4640–4645. doi: 10.1021/nl5016975. [DOI] [PubMed] [Google Scholar]
- 122.Scudellari B.M. The pandemic’s future. Nature. 2020:1–4. https://www.nature.com/articles/d41586-020-02278-5 [Google Scholar]