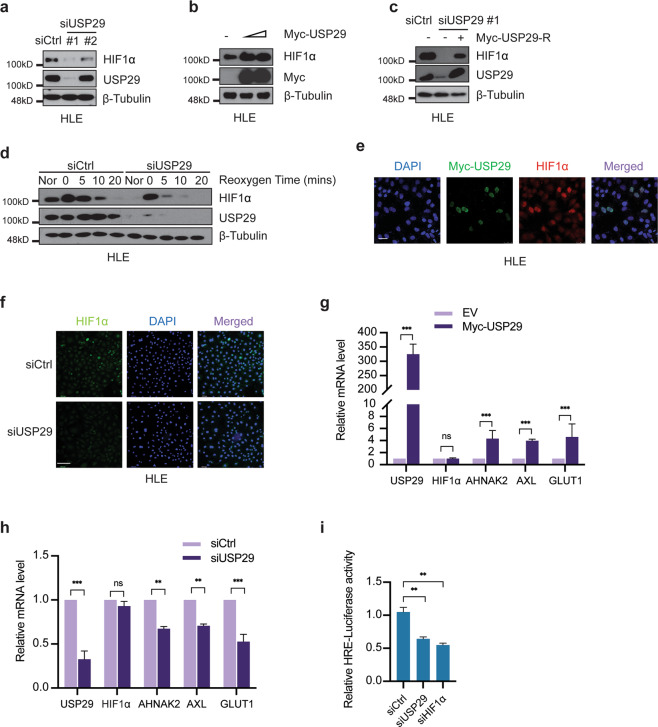
Fig. 2. USP29 stabilizes HIF1α and promotes HIF1α’s transcriptional activity.
a Depletion of USP29 diminished HIF1α protein levels in Sorafenib-resistant HLE cells. Two different siRNAs against USP29 (siUSP29#1 and siUSP29#2) were transfected into cells for the depletion of USP29. siUSP29#1 had a superior knockdown efficiency than siUSP29#2. Based on its high knockdown efficiency, siUSP29#1 was used for further experiments. Immunoblotting for β-Tubulin was used as loading control. Results represent three independent experiments. b USP29 promotes HIF1α protein stability. Myc-tagged USP29 was transfected into HLE cells, and endogenous HIF1α protein level was measured by immunoblotting. Immunoblotting for β-Tubulin was used as loading control. Results represent three independent replicative experiments. c Expression of an RNAi-resistant USP29 (Myc-USP29-R) rescued USP29 deficiency-induced instability of HIF1α. HLE cells were first transfected with siUSP29#1 and 24 h later with Myc-USP29-R or Empty-Vector. USP29 and HIF1α protein levels were determined by immunoblotting. Immunoblotting for β-Tubulin was used as loading control. Results represent three independent replicative experiments. d USP29 deficiency induces HIF1α protein degradation. HLE cells transfected with siCtrl or ON-TARGET siUSP29 were incubated in a hypoxia chamber (1% O2, 94% N2, 5% CO2) for 6 h and then moved to normoxia for 0, 5, 10, and 20 min. Culture in normoxia (Nor) was used as a control. Immunoblotting was used to visualize the kinetics of HIF1α degradation. Immunoblotting for USP29 was used to validate the knockdown efficiency and β-Tubulin as loading control. Results represent three independent replicative experiments. e USP29 promotes HIF1α stability and nuclear localization. A plasmid encoding for Myc-tagged USP29 was transfected into HLE cells and Myc-tagged USP29 and endogenous HIF1α were visualized by immunofluorescence microscopy analysis. Staining with DAPI was used to visualize nuclei. Results represent three independent experiments. Scale bar, 50 µm. f Loss of USP29 expression reduces HIF1α stability and nuclear localization. HLE cells were transfected with siCtrl or ON-TARGET siUSP29, and HIF1α was visualized by immunofluorescence microscopy for staining of endogenous HIF1α. DAPI staining was used to visualize nuclei. Scale bar, 132.5 µm. g USP29 promotes HIF1α transcriptional activity. Expression of the HIF1α target genes AHNAK2, AXL, GLUT1 was examined in HLE cells transfected with a plasmid encoding for Myc-tagged USP29, and mRNA levels were determined by quantitative RT-PCR. Relative mRNA expression is shown. n = 3 independent replicates. ns = not significant; ***P < 0.001; Student’s t-test. h USP29 deficiency reduces HIF1α transcriptional activity. HLE cells were transfected with siCtrl or ON-TARGET siUSP29, and the expression of a panel of HIF1α transcriptional target genes was analyzed by quantitative RT-PCR. Relative mRNA expression is shown. n = 3 independent replicates. ns not significant; **P < 0.01; ***P < 0.001; Student’s t-test. i Loss of USP29 expression reduces HIF1α transcriptional activity. HLE cells were transfected with siCtrl, ON-TARGET siUSP29, and siHIF1α and 24 h later with plasmids carrying a HIF responsive element (HRE) driving the expression of Firefly luciferase (pGL4.42) and CMV promoter-driven Renilla luciferase (pRL-CMV) in a 10:1 mass ratio. Relative luciferase activities were measured by a dual-luciferase reporter assay. Results represent three independent experiments. **P < 0.01; Student’s t-test.