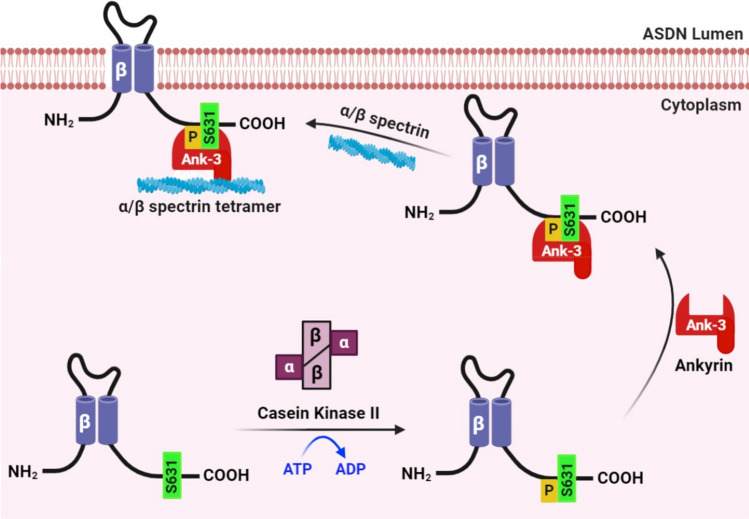
Figure 7.
CKII increases ENaC activity by phosphorylating β-ENaC within its anchor motif to promote Ank-3 mediated trafficking to the membrane. Proposed mechanism by which CKII modifies ENaC activity. Our hypothesis is that phosphorylation by CKII of a key serine residue in a consensus CKII site contained within a canonical anchor motif within the COOH-terminal domain of β-ENaC is necessary and sufficient for the channel to bind Ank-3 with the latter being necessary for appropriate channel locale and activity, which is required for the physiological function of the channel and the appropriate fine-tuning of renal Na+ excretion. Created with BioRender.com.