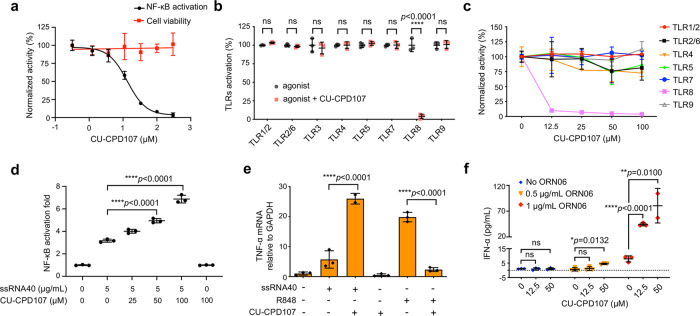
Fig. 3. Dichotomous behavior of CU-CPD107 in modulating the TLR8 signaling pathway.
a The dose–response inhibition curve of 2.9 µM R848-induced signaling in HEK-Blue hTLR8 cells is shown. Inhibition is clearly observed in HEK-Blue hTLR8 cells (IC50 = 13.7 ± 1.1 µM) with no obvious toxicity up to 300 μM. Data are mean ± s.d.; n = 3 independent experiments. b Specificity test for CU-CPD107 (100 µM) using TLR-specific ligands to selectively activate the respective TLRs in HEK-Blue hTLRs cells for 24 h. Data are mean ± s.d.; n = 3 independent experiments. c Specificity test of CU-CPD107 in PBMCs. Except for TLR8, CU-CPD107 alone did not inhibit other TLR pathways. Data are normalized to CU-CPD107 untreated cells. Data are mean ± s.d.; n = 3 independent experiments with independent blood donors. d CU-CPD107 could also synergistically activate TLR8 signaling in the presence of ssRNA40 (5 μg/mL) with a dose-dependent manner, while CU-CPD107 alone was unable to induce any activation. Signals are normalized to the untreated cells. Data are mean ± s.d.; n = 3 independent experiments. e CU-CPD107 could synergistically upregulate the mRNA levels of TNF-α in the presence of ssRNA40 (5 μg/mL), while it could inhibit R848-induced activation and itself had no effect in HEK-Blue hTLR8 cells. Data are mean ± s.d.; the data shown are representative of three biologically independent experiments. f The synergistic effect of CU-CPD107 in human PBMCs stimulated with ORN06. The production of IFN-α was measured by ELISA. Data are mean ± s.d.; the data shown are representative of three samples of independent blood donors examined over three biologically independent experiments. A one-way analysis of variance with Bonferroni’s multiple comparisons test for multiple comparisons was used for statistical analysis. Statistical significance of the data is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns not significant. Source data of Fig. 3 are provided as a Source data file.