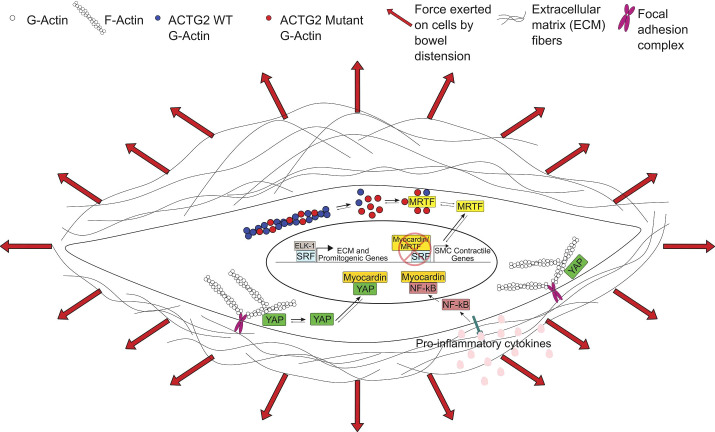
Figure 1.
Effects of actin gamma 2 (ACTG2) mutations and inflammation on transcriptional regulation in visceral myopathy. We propose that ACTG2 variants polymerize less efficiently than wild-type (WT) ACTG2, resulting in increased monomeric globular actin (G-actin). The increased G-actin may sequester myocardin-related transcription factors (MRTFs) in the cytoplasm, downregulating smooth muscle cell (SMC) contractile gene expression. With less MRTF in the nucleus, serum response factor (SRF) is free to bind with ELK-1, leading to increased expression of extracellular matrix (ECM) and promitogenic genes. Increased ECM deposition leads to a stiffer SMC microenvironment. This increase in stiffness along with pathologic levels of stretch due to abnormal bowel or bladder distension may increase tension on focal adhesions and release Yes-associated protein (YAP), allowing YAP to translocate to the nucleus. Nuclear YAP inhibits the interaction of myocardin with SRF, further downregulating the SMC contractile phenotype. Finally, NF-κB activation downstream of proinflammatory cytokines may further inhibit SMC contractile gene expression by NF-κB-myocardin binding. F-actin, filamentous actin.