Figure 3.
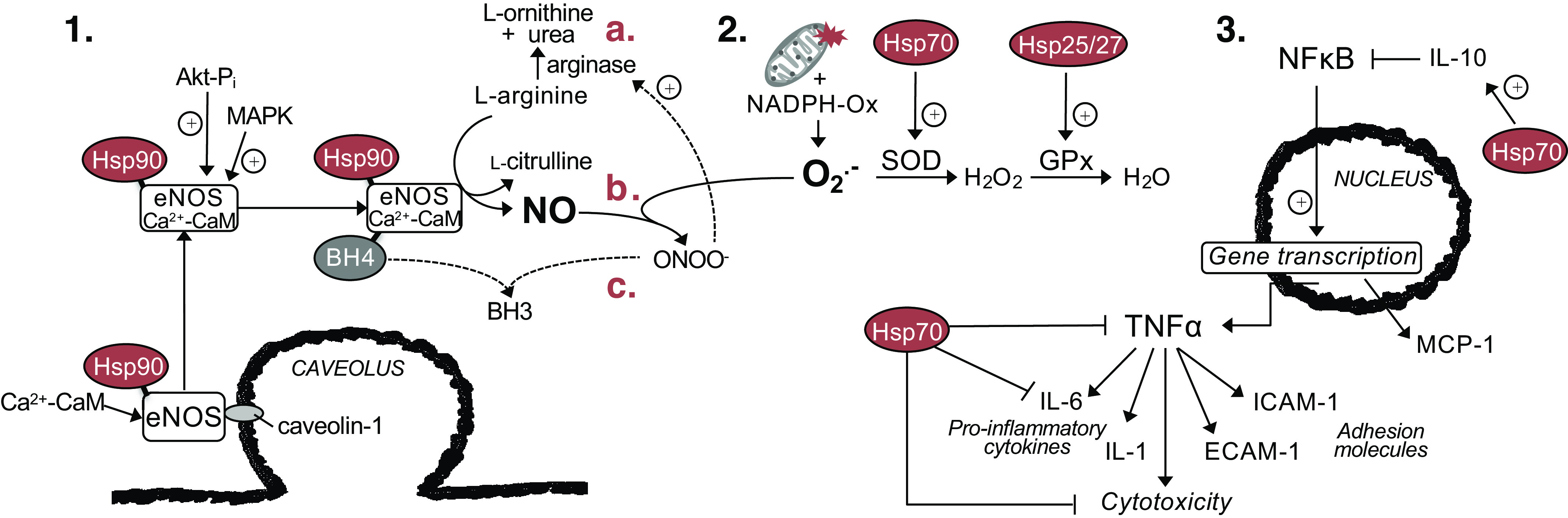
Interactions of heat shock proteins (HSPs) in endothelial cells with three primary pathways associated with vascular function: the nitric oxide (NO) pathway, oxidative stress, and inflammation. 1: Hsp90 is essential for activation of endothelial NO synthase (eNOS) by calcium-calmodulin (Ca2+-CaM) and Akt (also known as protein kinase B). 2: Hsp70 upregulates superoxide dismutase (SOD) and Hsp25/27 upregulates glutathione peroxidase (GPx), such that the damaging effects of reactive oxygen species are attenuated. These damaging effects include a) upregulation of arginase, which then decreases available l-arginine for synthesis of NO; b) scavenging of NO; and c) scavenging of tetrahydrobiopterin (BH4), and thus uncoupling of eNOS. 3: Heat stress, most likely via Hsp70, suppresses nuclear factor-kappa-B (NF-κB), a master regulator of proinflammatory gene transcription, and its downstream effects mediated by proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6. Hsp70 also upregulates the anti-inflammatory cytokine IL-10, which can suppress NF-κB activation. NADPH-Ox, nicotinamide adenine dinucleotide phosphate oxidase; MAPK, mitogen-activated protein kinase; ONOO-, peroxynitrite; MCP-1, monocyte chemoattractant protein-1; ICAM-1, intercellular adhesion molecule-1; ECAM-1, endothelial cell adhesion molecule-1.
