Abstract
Diminished cerebrovascular function is associated with reduced cognitive ability. Habitual exercise may maintain or improve cerebrovascular function; however, limited information exists regarding the optimal exercise prescription for cerebrovascular health. Although aerobic exercise is associated with improved systemic vascular function, the influence of resistance exercise on vascular health is unclear. Therefore, the purpose of this study was to examine the influence of habitual exercise training on cerebrovascular function in healthy young adults. We evaluated 13 untrained (age = 27 ± 5 yr; 11 men, 2 women), 13 aerobic-trained (age = 28 ± 5 yr; 10 men, 3 women), and 13 resistance-trained (age = 24 ± 4 yr; 11 men, 2 women) adults. Middle cerebral artery velocity (MCAv), mean arterial pressure (MAP), and end-tidal carbon dioxide were continuously measured at rest and in response to hypercapnia. At rest, there were no differences between groups for MCAv, however, resistance-trained adults had greater cerebrovascular conductance compared with aerobic-trained adults (0.79 ± 0.26 cm/s/mmHg vs. 0.56 ± 0.17 cm/s/mmHg; P < 0.05). In response to hypercapnia, cerebrovascular reactivity and MAP reactivity were not different between groups. There was no association between aerobic fitness or measures of exercise volume and any variable of cerebrovascular function in the combined or individual groups. Our results suggest that the mode of exercise training does not impact cerebrovascular reactivity in healthy young adults, however, it may influence resting cerebral hemodynamics. Future research could examine the influence of habitual exercise training on cerebrovascular function with aging.
NEW & NOTEWORTHY Habitual exercise may influence cerebral hemodynamics, as it affects other variables of vascular health in this population. We report that habitual exercise training does not influence cerebrovascular reactivity in young adults, as there were no significant differences between aerobic-trained, resistance-trained, and untrained individuals. Despite this finding, the mode of habitual exercise training had a moderate influence on resting cerebral hemodynamics such that resistance-trained adults had greater cerebrovascular conductance compared with aerobic-trained adults.
Keywords: cerebral blood flow, exercise training, physical activity, resistance training, vascular health
INTRODUCTION
The cerebral circulation is important for maintaining overall brain health, as poor cerebrovascular function is associated with stroke and cognitive decline in older adults (1). Cerebrovascular health starts to decline during midlife and many lifestyle interventions are aimed at slowing this decline (1). Yet, greater cerebrovascular health during young adulthood may associate with better cerebrovascular health in midlife, due to having a higher baseline cerebrovascular function before the onset of age-associated declines in cerebrovascular function (2). Cerebrovascular reactivity to hypercapnia is a common method to assess cerebrovascular function, as it is associated with important clinical outcomes in middle-aged and older adults, such as risk of stroke and cognitive decline (3, 4). Although cerebrovascular reactivity is generally high in young adults, a greater cerebrovascular reactivity during young adulthood may be associated with better cognitive health in older adulthood (5, 6).
Habitual exercise may be one way to improve cerebrovascular health during young adulthood, however, studies examining the influence of habitual exercise or aerobic fitness on cerebrovascular reactivity have reported conflicting results (7–10) and many studies assessing the influence of habitual aerobic exercise on cerebrovascular reactivity have only done so in middle-aged or older adults (e.g., see Refs. 10–12). Furthermore, no study has examined the effects of habitual resistance training on cerebrovascular reactivity. Importantly, previous studies have shown that aerobic and resistance exercise produce different vascular adaptations (13–16), likely due to the nature of the hemodynamic stimulus during exercise (17, 18). Although acute aerobic exercise produces a relatively consistent increase in blood flow and shear stress along the vessel wall, acute resistance exercise can induce large spikes in blood pressure during concentric contractions, and therefore has a much more varied blood flow and shear stress pattern during exercise (17, 18). The consistent increase in blood flow and shear stress is likely why aerobic exercise is generally associated with beneficial vascular remodeling, as it is linked with improved endothelial function and central arterial stiffness (19). In addition, studies have shown a positive association between cardiorespiratory fitness and cerebrovascular reactivity to CO2 in young (7) and older adults (7, 8). In contrast, fewer studies have examined the effects of resistance exercise on vascular health and have often reported conflicting results across variables of central arterial stiffness and peripheral vascular function (18, 20).
Due to the existing gaps in the literature and the potential for habitual exercise during young adulthood to influence cerebrovascular health, the purpose of this study was to examine cerebrovascular function, determined as cerebrovascular reactivity to CO2, in healthy young adults who were either aerobic trained, resistance trained, or untrained. Due to the hemodynamic patterns present during aerobic and resistance exercise and because systemic vascular function (central arterial stiffness and endothelial function) appears to be influenced by exercise modality, we hypothesized that: 1) aerobic-trained adults would have greater cerebrovascular reactivity compared with both resistance trained and untrained adults; and 2) due to the inconsistent blood flow and patterns during resistance exercise, there would be no difference in cerebrovascular reactivity between resistance-trained adults and untrained adults. As a secondary aim, we sought to evaluate the potential association between aerobic fitness or exercise volume with cerebrovascular reactivity to explore the influence of aerobic fitness and training volume.
METHODS
Participants
Thirty-nine young adults, between 18 and 35 yr, were classified as either aerobic trained (n = 13 adults), resistance trained (n = 13 adults), or untrained (n = 13 adults). Participants were free from any known cardiovascular, neurological, hepatic, renal, hematological, or metabolic diagnoses. In addition, individuals with a history of tobacco or nicotine use were excluded. All female participants were studied in the early follicular phase of their menstrual cycle to minimize the effects of sex hormones. Classification of exercise status occurred through the use of questionnaires and detailed exercise training logs (7-day training log and the Godin Leisure-Time Exercise Questionnaire), and maximal aerobic capacity (V̇o2max) was measured on a cycle ergometer. Participants were considered untrained if they performed < 1 h of planned exercise per week for the previous 1 yr. The resistance-trained group consisted predominately of personal trainers from the surrounding community and were required to have performed structured, high-intensity resistance training for a minimum of 3 days/wk for at least 1 yr. Short-duration aerobic exercise was allowed for resistance-trained individuals only as a warm-up before or cool down after resistance training. Aerobic-trained individuals were primarily runners, cyclists, and triathletes and were required to have performed at least 150 min of moderate to vigorous intensity aerobic exercise across a minimum of 3 days/wk for the previous year. Informed written and verbal consent were obtained from all participants. In addition, all study procedures had approval from the University of Wisconsin-Madison Institutional Review Board and were performed in accordance with the Declaration of Helsinki.
Blood Analysis
Venipuncture was performed during a separate laboratory visit after an overnight fast. Blood samples were tested for fasting glucose (Cardiocheck PA, PTS Diagnostics, Whitestown, IN), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and total cholesterol (Cardiocheck PA, PTS Diagnostics, Whitestown, IN).
Maximal Aerobic Capacity Test
All participants completed a V̇o2max test during a separate laboratory visit. The test was performed on a Lode cycle ergometer (Excalibur Sport, Lode, Groningen, The Netherlands) and consisted of 1 min stepped increases of 20–40 W, until exhaustion. During exercise, respiratory parameters (Parvomedics True One 2400, Sandy, UT) and heart rate from a 12-lead electrocardiogram (CASE, GE Healthcare, Waukesha, WI) were continuously measured. Two of three criteria were met to qualify as an acceptable test: a maximal heart rate within 10 beats/min of the individual’s age predicted maximum heart rate (220-age); a respiratory exchange ratio (RER) greater than 1.10; or a plateau in V̇o2 despite increasing workload.
Experimental Protocol
Participants reported to the laboratory on the morning of the study day after an overnight fast and after having abstained from exercise, caffeine, and alcohol for 24 h, in addition to avoiding the use of nonsteroidal anti-inflammatory drugs for the previous 5 days. After arriving to the laboratory, height and weight were recorded. Once supine, brachial blood pressure was measured in triplicate, and participants were instrumented with a 3-lead electrocardiogram (Cardiocap 5, Datex-Ohmeda, Louisville, CO), nasal cannula (Cardiocap 5, Datex-Ohmeda, Louisville, CO), and a finger blood pressure cuff (Finapres Medical System, Amsterdam, The Netherlands) to continuously measure heart rate, end-tidal CO2 (), and beat-to-beat blood pressure, respectively. All physiological measures were collected at 250 Hz, interfaced with a WinDaq data acquisition system (Dataq Instruments, Akron OH), and recorded for offline analysis.
Assessment of Cerebrovascular Function
Middle cerebral artery blood flow velocity (MCAv) was measured through transcranial Doppler (TCD) ultrasound (ST3, Spencer Technologies, Redmond, WA) via a 2-MHz probe positioned at participant’s right transtemporal window and held in place with a headband. To determine cerebrovascular reactivity to hypercapnia, a stepped hypercapnia protocol was performed as described previously (21, 22). Briefly, participants breathed room air followed by consecutive elevations of medical grade gases, containing 2%, 4%, and 6% CO2 for 3 min each. The gases were administered through a mask with a one-way valve to prevent rebreathing (7450 V2, Hans Rudolph, Shawnee Mission, KS).
Data Analysis and Statistics
Beat-to-beat MCAv and MAP and breath-by-breath were averaged over the last minute of each level of the stepped hypercapnia protocol (room air, 2 % CO2, 4 % CO2, 6 % CO2). Cerebrovascular conductance index (CVCi) was calculated as MCAv/MAP. Both raw and percent changes in MCAv, CVCi, and MAP were assessed to calculate reactivity as the linear slope of the relationship between each respective variable and .
Power analysis.
To our knowledge, no study has directly compared the effects of different modalities of habitual exercise training on cerebrovascular reactivity, however, many cross-sectional studies have examined the effects of habitual exercise on vascular structure or function. Bailey et al. (7) examined cerebrovascular reactivity to CO2 in aerobic trained and sedentary young men. Based on the results of their study (aerobic trained: 3.78 ± 0.84 %/mmHg, n = 20 men; untrained: 2.10 ± 0.73 %/mmHg, n = 19 men), a total sample size of 10 (n = 5 participants per group) would yield a power of 80%. In addition, no study has examined the influence of habitual resistance training on cerebrovascular reactivity, however, Otsuki et al. (23) examined central arterial adaptations (carotid-femoral pulse wave velocity) in young men who were either resistance trained (∼6.5 ± 0.3 m/s), endurance trained (∼5.5 ± 0.3 m/s), or sedentary (∼5.9 ± 0.3 m/s). Based on these results, we calculated a total sample size of 33 participants (n = 11 per group) would yield a power of 80%. From these studies, we determined that a group of n = 13 participants would present a reasonable balance between statistical power and the cost of additional participant recruitment and testing.
Statistical analysis.
Statistics were performed using SigmaPlot statistical software (SYSTAT Software, San Jose, CA), with the exception of effect size, which was calculated using G*Power (24). Normality of data was determined through Shapiro–Wilk tests, and equal variance was tested with Brown–Forsythe tests. Comparisons between groups (participant characteristics, hemodynamics, and cerebrovascular function) were performed using one-way analysis of variance (ANOVA) tests, with supplemental pairwise comparisons performed using the Holm–Sidak test. Within-group responses to hypercapnia were analyzed using repeated measures one-way ANOVA tests, with supplemental pairwise comparisons performed using the Holm–Sidak test. Associations were determined through Pearson product-moment correlations. Effect size was calculated as Cohen’s f. In all cases, results are represented as means ± SD, and a P value < 0.05 was considered statistically significant.
RESULTS
Participant characteristics are shown in Table 1. Although the groups were similar in age, height, weight, blood pressure, and blood markers, aerobic-trained adults had a lower resting heart rate when compared with untrained individuals (Table 1). As expected, aerobic-trained adults had a greater V̇o2max when compared with both untrained and resistance-trained participants (P < 0.01 for both; Table 2), with resistance-trained individuals having a greater V̇o2max than untrained participants (P < 0.01; Table 2). By design, compared with untrained adults, aerobic-trained and resistance-trained individuals performed more physical activity per week, demonstrated by min/wk of their respective activities (P < 0.001 for both; Table 2) and Godin scores (P < 0.05 for both; Table 2). In addition, aerobic-trained adults had greater metabolic equivalent of task (MET)-minutes/ week of physical activity compared with untrained individuals (P < 0.01); however, the difference in calculated MET-minutes/week between resistance-trained individuals and untrained individuals did not reach significance (P = 0.08).
Table 1.
Participant characteristics
| Variable | Untrained | Aerobic Trained | Resistance Trained | P Value |
|---|---|---|---|---|
| n, men/women | 13, 11/2 | 13, 10/3 | 13, 11/2 | |
| Age, yr | 27 ± 5 | 28 ± 5 | 24 ± 4 | 0.11 |
| Height, cm | 175 ± 7 | 174 ± 9 | 179 ± 6 | 0.18 |
| Weight, kg | 76 ± 11 | 73 ± 12 | 79 ± 7 | 0.23 |
| Body mass index, kg/m2 | 25 ± 2 | 24 ± 2 | 25 ± 2 | 0.52 |
| Resting heart rate, beats/min | 59 ± 8 | 51 ± 6* | 56 ± 9 | 0.03 |
| Systolic blood pressure, mmHg | 117 ± 9 | 119 ± 12 | 124 ± 9 | 0.14 |
| Diastolic blood pressure, mmHg | 68 ± 5 | 70 ± 8 | 71 ± 6 | 0.52 |
| Mean blood pressure, mmHg | 84 ± 6 | 86 ± 8 | 89 ± 6 | 0.26 |
| Fasting glucose, mg/dL | 77 ± 9 | 78 ± 9 | 70 ± 7 | 0.05 |
| Total cholesterol, mg/dL | 141 ± 38 | 156 ± 29 | 144 ± 44 | 0.59 |
| High-density lipoprotein, mg/dL | 46 ± 8 | 59 ± 19 | 54 ± 12 | 0.06 |
| Low-density lipoprotein, mg/dL | 85 ± 32 | 85 ± 29 | 82 ± 48 | 0.98 |
Values are means ± SD. *P ≤ 0.05 vs. untrained.
Table 2.
Aerobic fitness and training log data
| Variable | Untrained (n = 13) | Aerobic Trained (n = 13) | Resistance Trained (n = 13) | P Value |
|---|---|---|---|---|
| Min/wk RE | 9 ± 23 | 14 ± 28 | 350 ± 184† | <0.001 |
| Min/wk moderate AE | 12 ± 34 | 126 ± 107† | 10 ± 20 | <0.001 |
| Min/wk vigorous AE | 30 ± 64 | 174 ± 130† | 7 ± 18 | <0.001 |
| V̇o2max, mL/kg/min | 34 ± 7 | 50 ± 5† | 42 ± 5* | <0.001 |
| Max workload, W | 227 ± 60 | 334 ± 55* | 316 ± 38* | <0.001 |
| MET-min/wk | 1,888 ± 1,270 | 4,049 ± 1,441* | 3,207 ± 1,971 | <0.01 |
| Godin score, AU | 36 ± 19 | 64 ± 17* | 55 ± 19* | <0.01 |
Values are means ± SD. AE, aerobic exercise; MET, metabolic equivalent of task; RE, resistance exercise; V̇o2max, maximal oxygen uptake. Exercise volumes were derived from 7-day training logs filled out by study participants. *P ≤ 0.05 vs. untrained adults; †P ≤ 0.05 vs. all.
The physiological response to hypercapnia increased MCAv, MAP, and CVCi in all groups from room air to 6% CO2 (P ≤ 0.01 for all; Table 3). In addition, all groups reached a similar at each level of hypercapnia (Table 3). Resistance-trained individuals had a greater MCAv at 2% hypercapnia than aerobic-trained individuals (Table 3). At baseline and during 2% hypercapnia, resistance-trained individuals had a greater CVCi compared with aerobic-trained individuals (Table 3). There were no differences between groups for cerebrovascular reactivity, measured as raw MCAv reactivity slope, or MAP reactivity (Fig. 1). In addition, there was no differences between groups when cerebrovascular reactivity was analyzed as the slope between the percent change in and the percent change in MCAv (untrained: 1.10 ± 0.30 %cm/s/%mmHg; aerobic trained: 1.51 ± 0.58 %cm/s/%mmHg; resistance trained: 1.44 ± 0.92 %cm/s/%mmHg; overall P = 0.24; f = 0.30). There were also no differences in cerebrovascular reactivity between the combined aerobic- and resistance-trained groups and the untrained group as a raw MCAv slope (untrained: 1.71 ± 0.60 cm/s/mmHg; trained: 2.18 ± 1.26 cm/s/mmHg; P = 0.21; f = 0.24) or % change slope (untrained: 1.10 ± 0.30 %cm/s/%mmHg, trained: 1.47 ± 0.75 %cm/s/%mmHg; P = 0.09, f = 0.34). Furthermore, when cerebrovascular reactivity was evaluated using CVCi reactivity, there were also no differences between groups when analyzed as a raw slope (untrained: 0.017 ± 0.007 cm/s/mmHg2, aerobic trained: 0.019 ± 0.010 cm/s/mmHg2, resistance trained: 0.015 ± 0.019 cm/s/mmHg2; P = 0.77; f = 0.13) or % change slope (untrained: 0.95 ± 0.32 %cm/s/%mmHg, aerobic trained: 1.34 ± 0.54 %cm/s/%mmHg, resistance trained: 0.98 ± 0.92 %cm/s/%mmHg; P = 0.24; f = 0.30). Lastly, there were no associations between V̇o2max or measures of training volume and cerebrovascular reactivity or MAP reactivity in individual groups or the combined participant population (Table 4).
Table 3.
Hemodynamic and gas exchange variables during stepped hypercapnia
| Variable | Untrained (n = 13) | Aerobic Trained (n = 13) | Resistance Trained (n = 13) | P Value | Cohen’s f |
|---|---|---|---|---|---|
| MCAv, cm/s | |||||
| Room air | 61 ± 16 | 54 ± 16 | 71 ± 20 | 0.06 | 0.39 |
| 2% CO2 | 64 ± 17 | 56 ± 16 | 74 ± 19* | 0.05 | 0.41 |
| 4% CO2 | 72 ± 15† | 66 ± 19† | 81 ± 19† | 0.13 | 0.34 |
| 6% CO2 | 81 ± 18† | 75 ± 23† | 91 ± 22† | 0.18 | 0.31 |
| CVCi, cm/s/mmHg | |||||
| Room air | 0.69 ± 0.19 | 0.56 ± 0.17 | 0.79 ± 0.26* | 0.03 | 0.46 |
| 2% CO2 | 0.72 ± 0.19 | 0.57 ± 0.17 | 0.81 ± 0.24* | 0.02 | 0.50 |
| 4% CO2 | 0.80 ± 0.19† | 0.67 ± 0.18† | 0.85 ± 0.22 | 0.06 | 0.40 |
| 6% CO2 | 0.88 ± 0.20† | 0.74 ± 0.22† | 0.93 ± 0.22† | 0.07 | 0.38 |
| MAP, mmHg | |||||
| Room air | 90 ± 13 | 99 ± 13 | 92 ± 11 | 0.18 | 0.30 |
| 2% CO2 | 90 ± 13 | 100 ± 12 | 92 ± 11 | 0.10 | 0.35 |
| 4% CO2 | 91 ± 13 | 100 ± 12 | 95 ± 11 | 0.19 | 0.30 |
| 6% CO2 | 93 ± 14† | 102 ± 12† | 98 ± 15† | 0.26 | 0.27 |
| , mmHg | |||||
| Room air | 39 ± 7 | 41 ± 5 | 42 ± 5 | 0.39 | 0.23 |
| 2% CO2 | 41 ± 7 | 42 ± 5† | 44 ± 5 | 0.45 | 0.21 |
| 4% CO2 | 46 ± 4† | 47 ± 4† | 47 ± 4† | 0.89 | 0.08 |
| 6% CO2 | 51 ± 3† | 52 ± 3† | 51 ± 3† | 0.73 | 0.13 |
Values are means ± SD. CVCi, cerebrovascular conductance index; , end-tidal carbon dioxide; MAP, mean arterial pressure; MCAv, middle cerebral artery velocity. *P < 0.05 vs. aerobic-trained adults; †P < 0.05 vs. room air condition within each group.
Figure 1.
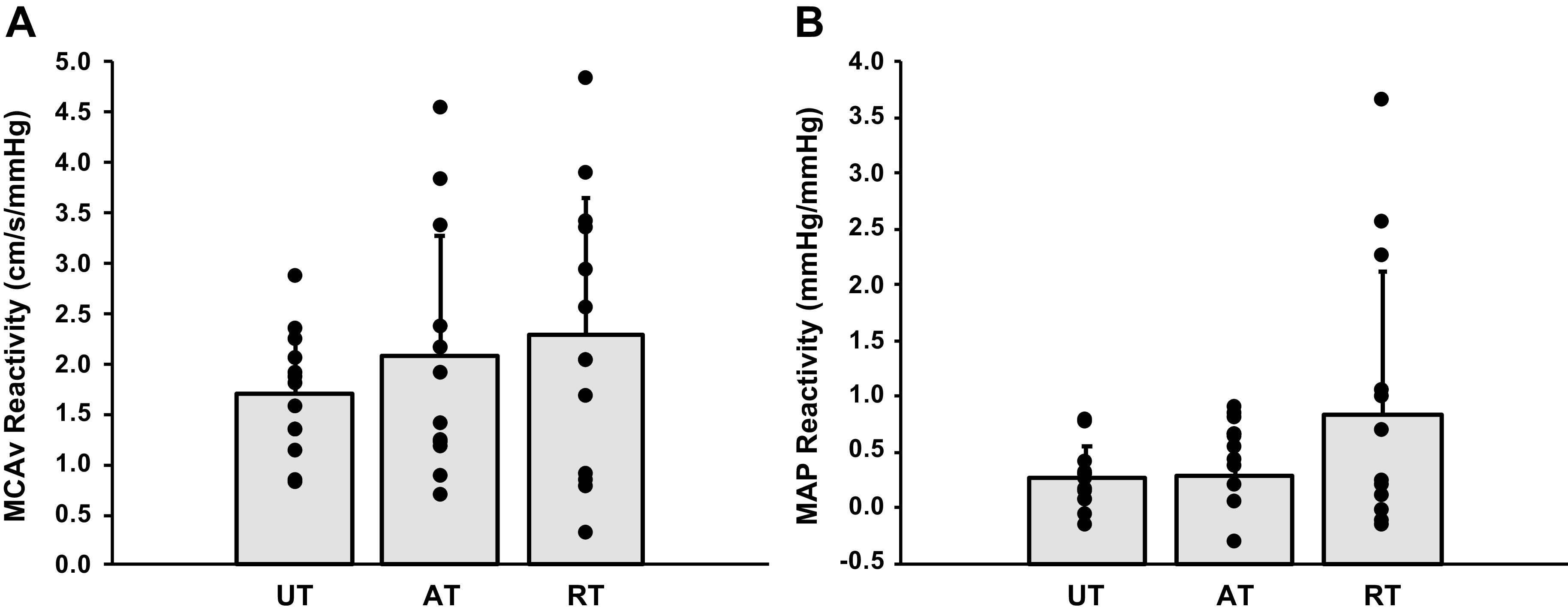
Data are means ± SD. MCAv reactivity (A) and MAP reactivity (B) are shown for aerobic-trained (AT; n = 13), resistance-trained (RT; n = 13), and untrained (UT; n = 13) participants. There were no significant differences in MCAv reactivity (overall P = 0.42, f = 0.22) or MAP reactivity (overall P = 0.12; f = 0.42) to hypercapnia between the three groups of young adults. MAP, mean arterial pressure; MCAv, middle cerebral artery velocity.
Table 4.
Associations between cerebrovascular reactivity and blood pressure reactivity with aerobic fitness and exercise volume
| MCAv Reactivity | CVCi Reactivity | MAP Reactivity | |
|---|---|---|---|
| All participants | |||
| V̇o2max | r = 0.15; P = 0.37 | r = 0.06; P = 0.71 | r = 0.02; P = 0.91 |
| MET-min/wk | r = 0.23; P = 0.16 | r = 0.15; P = 0.38 | r = 0.05; P = 0.76 |
| Godin | r = −0.06; P = 0.74 | r = −0.14; P = 0.41 | r = 0.05; P = 0.76 |
| Untrained | |||
| V̇o2max | r = −0.24; P = 0.43 | r = −0.34; P = 0.26 | r = 0.19; P = 0.53 |
| MET-min/wk | r = −0.05; P = 0.86 | r = −0.17; P = 0.59 | r = 0.27; P = 0.37 |
| Godin | r = 0.00; P = 0.99 | r = −0.19; P = 0.53 | r = 0.33; P = 0.27 |
| Aerobic trained | |||
| V̇o2max | r = 0.01; P = 0.98 | r = 0.06; P = 0.84 | r = −0.25; P = 0.41 |
| MET-min/wk | r = 0.15; P = 0.62 | r = 0.14; P = 0.65 | r = 0.10; P = 0.74 |
| Godin | r = −0.11; P = 0.73 | r = −0.19; P = 0.54 | r = −0.08; P = 0.80 |
| Resistance trained | |||
| V̇o2max | r = 0.37; P = 0.21 | r = 0.25; P = 0.41 | r = 0.06; P = 0.86 |
| MET-min/wk | r = 0.26; P = 0.39 | r = 0.23; P = 0.46 | r = −0.01; P = 0.96 |
| Godin | r = −0.34; P = 0.26 | r = −0.21; P = 0.50 | r = −0.03; P = 0.93 |
CVCi, cerebrovascular conductance index; Godin, Godin Leisure-Time Exercise Questionnaire; MAP, mean arterial pressure; MCAv, middle cerebral artery velocity; MET, metabolic equivalent of task; V̇o2max, maximal oxygen uptake. No significant associations.
DISCUSSION
To our knowledge, this is the first study to evaluate cerebrovascular reactivity in resistance-trained individuals and to directly compare across multiple modalities of habitual exercise training. Contrary to our hypothesis, we found no difference in cerebrovascular reactivity to hypercapnia between untrained, aerobic trained, or resistance-trained healthy young adults. We did, however, find that resistance-trained individuals had a greater cerebrovascular conductance at rest and during mild hypercapnia and a greater MCAv during mild hypercapnia compared with aerobic-trained adults. These results suggest that habitual exercise training does not influence cerebrovascular reactivity in healthy young adults, but may influence resting cerebral hemodynamics in this population.
Previous studies have shown opposing influences of aerobic and resistance exercise on variables of vascular health, which may be due to their contrasting hemodynamic profiles (13–18). Resistance exercise presents large pulsatile changes in blood flow and shear stress pattern throughout the exercise, as blood pressures can exceed 300 mmHg during the concentric portion of the exercise (25). Over time, regular exposure to suprasystolic blood pressures could potentially result in damage or unfavorable remodeling of the vasculature, which could have direct and/or indirect effects on the cerebral circulation. This remodeling is especially relevant when considering the delicate nature of the cerebral microvasculature (26, 27). This is in direct contrast to aerobic exercise, which often presents a relatively consistent pattern of augmented blood flow and shear stress throughout the duration of exercise (given that intensity remains similar) (17). These consistent elevations in shear stress have been shown to be beneficial for vascular remodeling and function, especially when vascular health is initially reduced (20).
In contrast to the present study, previous research has reported enhanced resting MCAv or cerebrovascular reactivity in aerobic-trained men (7, 28). However, our results support previous studies demonstrating that cerebral hemodynamics (MCAv, cerebrovascular reactivity, and dynamic cerebral autoregulation) do not differ between young aerobic trained and untrained individuals (9, 29). In addition, our data on untrained and aerobic-trained individuals is congruent with our previous work showing no association between cerebrovascular reactivity and aerobic fitness in young adults (8). These conflicting results, regarding the effect of habitual aerobic exercise on MCAv and cerebrovascular reactivity, may be due to the variety of physical activity participation and range of aerobic fitness levels in the recruited participants. For example, Bailey et al. (7). studied 39 young men with a wide range of V̇o2max values (∼25–85 mL/kg/min; untrained: 36 mL/kg/min, trained: 62 mL/kg/min) and reported a significant effect of habitual aerobic exercise and an association between aerobic fitness and cerebrovascular reactivity in the combined group. The range of V̇o2max values for the men and women in the present study was from 28 to 62 mL/kg/min with no difference in cerebrovascular reactivity between untrained individuals and habitual exercisers and no association between aerobic fitness and cerebrovascular function.
In the present study, young healthy adults were recruited to evaluate the effect of different modalities of habitual exercise training on cerebrovascular reactivity. A potential explanation for our finding that young, untrained adults had similar cerebrovascular function, as their exercise trained peers is that low levels of exercise may be adequate to prevent inactivity-associated cerebrovascular dysfunction in young adults. We recruited untrained individuals, defined as performing planned exercise for ≤ 1 day/wk, but did not consider the amount of nonexercise physical activity performed. Although the participants recruited for this study were untrained, 0–1 day/wk of planned exercise combined with adequate levels of nonexercise physical activity may be enough to protect against inactivity-associated cerebrovascular dysfunction in this healthy population of young adults. Adding support to this notion of vascular protection by nonexercise physical activity, intermittent slow walking is able to prevent inactivity-associated decline in MCAv and vascular dysfunction in young adults (30, 31). Because many of our participants were recruited from a college campus, the amount of intermittent nonexercise physical activity performed may explain why the cerebrovascular function of untrained adults was not different from the exercising groups.
As mentioned previously, to our knowledge, there are no studies that have evaluated the effect of regular resistance training on cerebrovascular reactivity. There are, however, studies that have examined the effects of resistance training on the central (aortic and carotid) and peripheral (limb) vasculature, which have yielded contrasting results (18–20). Previous studies have shown increased stiffness of the central elastic arteries in young men (18), but also improved endothelial function (flow-mediated dilation) in this same population (20); both of which may influence cerebrovascular function. Despite these findings, the cerebrovasculature of healthy young adults appears resilient against hemodynamic perturbations that would otherwise translate into the peripheral circulation; therefore, it may be necessary to quantify cerebral hemodynamics together with measures of central or peripheral hemodynamics. Studies that have examined the acute effects of resistance exercise in a young population on central and cerebrovascular hemodynamics have shown acute increases in central arterial stiffness with maintained cerebrovascular hemodynamics (32, 33). Interestingly, Rosenberg et al. (33) reported reduced cerebral blood flow regulation in an older population following acute resistance exercise. Therefore, the results of the present study may not transfer to middle-aged or older adults, as the acute impairment in cerebral blood flow regulation reported by Rosenberg et al. may result in cerebrovascular dysfunction with habitual resistance training.
The results of the present study have important implications for exercise prescription with the intent of improving cerebrovascular and cognitive health. Although we found no significant difference in cerebrovascular function (cerebrovascular reactivity) between untrained, aerobic-trained, and resistance-trained individuals, we reported that resistance-trained individuals had greater resting cerebrovascular conductance compared with aerobic trained individuals. It is unclear as to what possible mechanisms may be behind the greater CVCi of the resistance-trained group, however, resistance training programs vary widely and often involve different movements/stimuli, whereas aerobic training largely induces a similar movement pattern with only the intensity differing. Although we specifically recruited participants who performed high-intensity resistance training at least 3 days/wk, there is a large variation as to what movements and programs that are encompassed under high-intensity resistance training. This variability might also explain the objectively larger range of values in our MCAv reactivity and MAP reactivity data in the resistance-trained adults compared with the aerobic trained and untrained groups. Nonetheless, our finding of a greater CVCi in resistance-trained adults is consistent with the finding of Stebbings et al. (34), who found that carotid artery diameter and flow were increased after an 8-wk resistance training program. Although not significant, the objectively greater MAP reactivity of the resistance-trained group may be of interest for future studies, especially as the overall effect size for MAP reactivity across groups was large (f = 0.42). We have previously reported similar cerebrovascular reactivity, but greater MAP reactivity in older adults compared with young adults (35). Follow-up studies should examine if resistance-trained individuals are more reliant on mechanisms observed regularly in older adults to increase cerebral blood flow and to determine if the other results of the present study are consistent in a middle-aged or older population.
This study was not without limitations, including: 1) due to the cross-sectional study design, resistance and aerobic-trained individuals likely had different training histories compared with other individuals within their respective groups. Although each exercise-trained individual participated in their respective activity for at least 1 yr, the activities, training intensities, and durations across individuals differed; 2) using self-reported measures of physical activity participation to deem eligibility is also a limitation of the study. Consequentially, participants may have inaccurately estimated their activity levels, resulting in inappropriate study inclusion. Despite this limitation, we attempted to minimize variability in training history by asking for training history through many different methods and recruiting from established clubs and existing groups in the community. In addition, although the untrained participants on this study did report occasional exercise, it was not habitual. Sedentary living may be a condition of its own and may have complicated the results of the study; therefore, including untrained participants who would not be classified as sedentary allowed us to examine the effects of habitual exercise without having to delineate the influence of a sedentary lifestyle. Aerobic fitness of participants was evaluated to help ensure proper study group inclusion; 3) although V̇o2max was used to verify reported aerobic training in all participants, we did not include a similar resistance-based performance metric in all participants. This was due to safety concerns asking untrained or aerobic-trained individuals to perform maximal muscle strength tests without appropriate skill and training; 4) the small number of women represented in this study is a limitation, as there is a possibility for sex differences in training effects. Although many women participate in resistance training, it was difficult to recruit young women who met criteria for this study due to participation in concurrent training programs. Because of the low number of women, our study was underpowered to examine sex differences to address this potential confounding factor. Despite this limitation, the data from the women included in this study were not outliers and removal of female participants did not alter the results; 5) using TCD ultrasound to measure cerebral blood flow is a limitation, as it relies on the assumption that the middle cerebral artery does not vasodilate under physiological stressors. Recent studies using magnetic resonance imaging (MRI) have shown cerebral vasodilation of the middle cerebral artery during hypercapnia in young adults (36, 37). Yet, it remains unclear if the magnitude of cerebral vasodilation is influenced by exercise training or mode of exercise. Despite these MRI findings, TCD is still commonly used to evaluate cerebral blood flow responses to stimuli as it provides the temporal resolution to evaluate beat-to-beat changes in cerebrovascular hemodynamics (38).
In summary, this study sought to determine the influence of habitual exercise training, namely habitual aerobic and resistance training, on cerebrovascular reactivity. Contrary to our hypothesis, there were no differences in cerebrovascular reactivity to hypercapnia between groups. However, when comparing resistance-trained adults to aerobic-trained adults, resistance-trained adults had higher cerebrovascular conductance at rest and a greater MCAv and conductance during the first stage of hypercapnia. Lastly, we found no association between aerobic fitness and cerebrovascular reactivity to hypercapnia. Although we did not find a benefit of aerobic exercise on cerebrovascular reactivity in young adults, we also did not find that resistance training was detrimental to the cerebral vessels, and may even be beneficial for cerebrovascular health, as resistance-trained individuals had greater resting cerebrovascular conductance compared with aerobic-trained individuals. Future research should examine the influence of habitual exercise training on cerebrovascular function in an older population with a longer training history, as there may be a more apparent effect due to an interaction of aging and cumulative training within a specific exercise modality.
GRANTS
This work was supported by the National Institutes of Health Grant– HL118154 (J.N.B.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.N.B. conceived and designed research; A.T.C., A.J.H., K.B.M., and J.N.B. performed experiments; A.T.C., A.J.H., K.B.M., and J.N.B. analyzed data; A.T.C., K.B.M., and J.N.B. interpreted results of experiments; A.T.C. and J.N.B. prepared figures; A.T.C. and J.N.B. drafted manuscript; A.J.H., K.B.M., and J.N.B. edited and revised manuscript; A.T.C., A.J.H., K.B.M., and J.N.B. approved the final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the study participants.
REFERENCES
- 1.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill SJ, Friedenreich CM, Sajobi TT, Longman RS, Drogos LL, Davenport MH, Tyndall AV, Eskes GA, Hogan DB, Hill MD, Parboosingh JS, Wilson BJ, Poulin MJ. Association between lifetime physical activity and cognitive functioning in middle-aged and older community dwelling adults: results from the brain in motion study. J Int Neuropsychol Soc 21: 816–830, 2015. doi: 10.1017/S1355617715000880. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5: 347–360, 2004. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 4.Silvestrini M, Pasqualetti P, Baruffaldi R, Bartolini M, Handouk Y, Matteis M, Moffa F, Provinciali L, Vernieri F. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke 37: 1010–1015, 2006. doi: 10.1161/01.STR.0000206439.62025.97. [DOI] [PubMed] [Google Scholar]
- 5.Davenport MH, Hogan DB, Eskes GA, Longman RS, Poulin MJ. Cerebrovascular reserve: the link between fitness and cognitive function? Exerc Sport Sci Rev 40: 153–158, 2012. doi: 10.1097/JES.0b013e3182553430. [DOI] [PubMed] [Google Scholar]
- 6.Peng S-L, Chen X, Li Y, Rodrigue KM, Park DC, Lu H. Age-related changes in cerebrovascular reactivity and their relationship to cognition: a four-year longitudinal study. Neuroimage 174: 257–262, 2018. doi: 10.1016/j.neuroimage.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, Ainslie PN. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44: 3235–3238, 2013. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- 8.Barnes JN, Taylor JL, Kluck BN, Johnson CP, Joyner MJ. Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J Appl Physiol 114: 1383–1387, 2013. doi: 10.1152/japplphysiol.01258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braz ID, Flück D, Lip GYH, Lundby C, Fisher JP. Impact of aerobic fitness on cerebral blood flow and cerebral vascular responsiveness to CO2 in young and older men. Scand J Med Sci Sports 27: 634–642, 2017. doi: 10.1111/sms.12674. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y-S, Tarumi T, Tseng BY, Palmer DM, Levine BD, Zhang R. Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and masters athletes. J Cereb Blood Flow Metab 33: 1190–1196, 2013. doi: 10.1038/jcbfm.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarumi T, Gonzales MM, Fallow B, Nualnim N, Lee J, Pyron M, Tanaka H, Haley AP. Cerebral/peripheral vascular reactivity and neurocognition in middle-age athletes. Med Sci Sports Exerc 47: 2595–2603, 2015. doi: 10.1249/MSS.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging 38: 1177–1183, 2013. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. doi: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 14.Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation 110: 2858–2863, 2004. doi: 10.1161/01.CIR.0000146380.08401.99. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto T, Masuhara M, Ikuta K. Effects of muscle contraction timing during resistance training on vascular function. J Hum Hypertens 23: 470–478, 2009. doi: 10.1038/jhh.2008.152. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 17.Green DJ, Hopman MTE, Padilla J, Laughlin MH, Thijssen DHJ. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97: 495–528, 2017. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med 47: 393–396, 2013. doi: 10.1136/bjsports-2012-090488. [DOI] [PubMed] [Google Scholar]
- 19.Seals DR, DeSouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol (1985) 105: 1323–1332, 2008. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, Mathers JC. Exercise modalities and endothelial function: a systematic review and dose–response meta-analysis of randomized controlled trials. Sports Med 45: 279–296, 2015. doi: 10.1007/s40279-014-0272-9. [DOI] [PubMed] [Google Scholar]
- 21.Barnes JN, Schmidt JE, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol 112: 1884–1890, 2012. doi: 10.1152/japplphysiol.01270.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie A, Skatrud JB, Morgan B, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol 577: 319–329, 2006. doi: 10.1113/jphysiol.2006.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Miyauchi T. Vascular endothelium-derived factors and arterial stiffness in strength- and endurance-trained men. Am J Physiol Heart Circ Physiol 292: H786–H791, 2007. doi: 10.1152/ajpheart.00678.2006. [DOI] [PubMed] [Google Scholar]
- 24.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 25.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol (1985) 58: 785–790, 1985. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell GF. Cerebral small vessel disease: role of aortic stiffness and pulsatile hemodynamics. J Hypertens 33: 2025–2028, 2015. doi: 10.1097/HJH.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 27.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 50: 1–13, 2007. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 28.Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJA, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586: 4005–4010, 2008. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry BG, Cotter JD, Korad S, Lark S, Labrecque L, Brassard P, Paquette M, Le Blanc O, Lucas SJE. Implications of habitual endurance and resistance exercise for dynamic cerebral autoregulation. Exp Physiol 104: 1780–1789, 2019. doi: 10.1113/EP087675. [DOI] [PubMed] [Google Scholar]
- 30.Carter SE, Draijer R, Holder SM, Brown L, Thijssen DHJ, Hopkins ND. Regular walking breaks prevent the decline in cerebral blood flow associated with prolonged sitting. J Appl Physiol (1985) 125: 790–798, 2018. doi: 10.1152/japplphysiol.00310.2018. [DOI] [PubMed] [Google Scholar]
- 31.Thosar S, Bielko S, Mather K, Johnston J, Wallace J. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc 47: 843–849, 2015. doi: 10.1249/MSS.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 32.Lefferts WK, Augustine JA, Heffernan KS. Effect of acute resistance exercise on carotid artery stiffness and cerebral blood flow pulsatility. Front Physiol 5: 101, 2014. doi: 10.3389/fphys.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg AJ, Schroeder EC, Grigoriadis G, Wee SO, Bunsawat K, Heffernan KS, Fernhall B, Baynard T. Aging reduces cerebral blood flow regulation following an acute hypertensive stimulus. J Appl Physiol 128: 1186–1195, 2020. doi: 10.1152/japplphysiol.00137.2019. [DOI] [PubMed] [Google Scholar]
- 34.Stebbings GK, Morse CI, McMahon GE, Onambele GL. Resting arterial diameter and blood flow changes with resistance training and detraining in healthy young individuals. J Athl Train 48: 209–219, 2013. doi: 10.4085/1062-6050-48.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller KB, Howery AJ, Harvey RE, Eldridge MW, Barnes JN. Cerebrovascular reactivity and central arterial stiffness in habitually exercising healthy adults. Front Physiol 9: 1096, 2018. doi: 10.3389/fphys.2018.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coverdale NS, Badrov MB, Shoemaker JK. Impact of age on cerebrovascular dilation versus reactivity to hypercapnia. J Cereb Blood Flow Metab 37: 344–355, 2017. doi: 10.1177/0271678X15626156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller KB, Howery AJ, Rivera-Rivera LA, Johnson SC, Rowley HA, Wieben O, Barnes JN. Age-related reductions in cerebrovascular reactivity using 4D flow MRI. Front Aging Neurosci 11: 281, 2019. doi: 10.3389/fnagi.2019.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoiland RL, Fisher JA, Ainslie PN. Regulation of the cerebral circulation by arterial carbon dioxide. Compr Physiol 9: 1101–1154, 2019. doi: 10.1002/cphy.c180021. [DOI] [PubMed] [Google Scholar]


