Abstract
The nucleus tractus solitarii (nTS) is the initial site of integration of sensory information from the cardiorespiratory system and contributes to reflex responses to hypoxia. Afferent fibers of the bilateral vagus nerves carry input from the heart, lungs, and other organs to the nTS where it is processed and modulated. Vagal afferents and nTS neurons are integrally associated with astrocytes and microglia that contribute to neuronal activity and influence cardiorespiratory control. We hypothesized that vagotomy would alter glial morphology and cardiorespiratory responses to hypoxia. Unilateral vagotomy (or sham surgery) was performed in rats. Prior to and seven days after surgery, baseline and hypoxic cardiorespiratory responses were monitored in conscious and anesthetized animals. The brainstem was sectioned and caudal, mid-area postrema (mid-AP), and rostral sections of the nTS were prepared for immunohistochemistry. Vagotomy increased immunoreactivity (-IR) of astrocytic glial fibrillary acidic protein (GFAP), specifically at mid-AP in the nTS. Similar results were found in the dorsal motor nucleus of the vagus (DMX). Vagotomy did not alter nTS astrocyte number, yet increased astrocyte branching and altered morphology. In addition, vagotomy both increased nTS microglia number and produced morphologic changes indicative of activation. Cardiorespiratory baseline parameters and hypoxic responses remained largely unchanged, but vagotomized animals displayed fewer augmented breaths (sighs) in response to hypoxia. Altogether, vagotomy alters nTS glial morphology, indicative of functional changes in astrocytes and microglia that may affect cardiorespiratory function in health and disease.
Keywords: autonomic nervous system, brainstem, cardiorespiratory, glia, hypoxia
INTRODUCTION
The nucleus tractus solitarii (nTS) is the initial site of integration of sensory information from the viscera, including the cardiorespiratory system (1). Afferent fibers of the bilateral vagus nerves carry input from the heart, lungs, and other organs to the nTS. There, this information is processed and modulated before being sent to brainstem and forebrain regions to regulate cardiorespiratory, gastrointestinal, endocrine, and other functions (1–3). The nTS is highly plastic; prolonged increases and decreases in afferent activity alter not only neurotransmission to the nTS but also the fundamental properties of nTS neurons (4–7). Although the mechanism(s) by which this activity-dependent plasticity occurs are not fully understood, glia have been shown to be integral to synaptic plasticity under a variety of physiological and pathophysiological conditions (2) and likely play a role.
Vagal afferents and nTS neurons are integrally associated with astrocytes, which together form the “tripartite synapse” (8, 9). Astrocytes are glial cells that are critical to maintaining the synaptic environment and regulating neuronal function (10). Astrocytes contribute to synaptic and neuronal activity and plasticity via release of gliotransmitters and neurotransmitter reuptake, including afferent-released glutamate (2, 10, 11). Vagal afferent stimulation activates nTS astrocytes (9) to induce gliotransmitter release and subsequent activation of adjacent neurons (12). Astrocyte glutamate uptake also critically controls nTS phasic and tonic glutamate concentrations, activation of glutamate receptors, and overall cardiorespiratory control (8, 13, 14). Furthermore, astrocytes exhibit state-dependent changes in their morphology that often indicate functional changes and may have dramatic effects on neurotransmission (11). Specifically, reactive astrocytes may express increased levels of glial fibrillary acidic protein (GFAP) and display increased or hypertrophied processes (11, 15). Within the paraventricular and supraoptic nuclei of the hypothalamus, dehydration and lactation alter astrocyte morphology (16) to influence neuronal function (17, 18).
In addition to astrocytes, microglia are abundant within the nTS (19). Microglia are typically considered the resident immune cells of the central nervous system. However, recent evidence suggests that microglia communicate with astrocytes, and also possess neurotransmitter receptors and reuptake transporters (20, 21). Microglia respond to changes in their environment, and shift from a ramified to amoeboid appearance when activated (20). Thus, together, glia likely contribute to modulation of nTS function and cardiorespiratory control (22, 23).
Given the activity-dependent plasticity of the nTS, and the contribution of astrocytes and microglia to neuronal activity in the nTS and other regions, our first goal was to determine the morphological alterations of these cells in response to changes in afferent input. To do so, we utilized unilateral vagus nerve transection (vagotomy) to disrupt normal afferent input to the nTS. This is clinically relevant as vagotomy may occur in heart and lung transplants and bariatric surgery in humans (24, 25). Previous studies have shown that vagotomy increases sensitivity of nTS neurons to glutamate (26) yet also decreases synaptic efficacy (27). The changes in nTS function post-vagotomy are likely complex and influenced by glia. We hypothesized that vagotomy augments branching in nTS astrocytes and shifts nTS microglia to an amoeboid appearance.
Our second goal was to examine the extent to which vagotomy and its altered glia are associated with changes in baseline cardiorespiratory function and responses to hypoxic stimulation. The vagus nerve (28) and glia (2, 29) contribute to physiological responses to periods of low oxygen (hypoxia). Hypoxia increases chemoafferent discharge through the glossopharyngeal nerve to augment nTS neuronal discharge, alters vagal activity, and enhances respiration and sympathetic nerve activity (28, 30–32). Enhanced respiration induced via chemoreflex activation increases pulmonary stretch receptor discharge whose afferents travel with the vagus nerves and modulate chemoreflex responses (28, 32). Within the nTS, sensory afferent terminal fields from several modalities overlap and have been suggested to converge onto nTS neurons to influence overall neuronal activity (33). Glia are also involved in hypoxic responses; prolonged hypoxia increases nTS microglial activation (19, 34) and several hypoxia-related diseases induce astrogliosis (34–37). We have also shown that nTS astrocyte glutamate transporters modulate chemoreflex function (8, 14).This suggests that glia play an important role in cardiorespiratory function and altered glial function may influence cardiorespiratory regulation. Nevertheless, the glial contribution to baseline respiration and reflex responses are not fully known. We hypothesized that in association with changes in glial morphology, vagotomy would influence cardiorespiratory responses to hypoxia.
METHODS
Animals and Ethical Approval
All animal protocols were performed in accordance with the American Physiological Society’s Guiding Principles for the Care and Use of Vertebrate Animals in Research and Training and the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the University of Missouri Animal Care and Use Committee. Male Sprague-Dawley rats (n = 48, Envigo, Indianapolis, IN) were allowed to acclimate in the vivarium for at least 1 wk before experimental procedures at 5–7 wk of age. Rats were cohoused in conventional caging with a 12:12 light:dark cycle (70–74°F, 30%–70% humidity) with food and water available ad libitum. Rats were randomly assigned to one of three groups: vagotomy (unilateral nerve transection), sham (surgery but no nerve transection), and control (no surgery).
Cardiorespiratory Monitoring
Conscious freely moving rats.
Breathing was monitored in awake, freely moving rats via whole body plethysmography as previously described (38). After a training period (3 days for 1 h each), rats were placed in flow-through (3 L/min) plethysmography chambers (Data Sciences International) with the animal and reference chambers connected to a differential pressure transducer (Validyne MP45; Validyne Engineering). Powerlab data acquisition system was used to obtain measurements and calculate respiratory rate, tidal volume index (normalized to body weight), and minute ventilation index (product of respiratory rate and tidal volume index). A HypoxyDial gas blender (STARR Life Sciences) was used to adjust inspired oxygen concentrations for normoxic baseline (21% O2) and hypoxic exposures (12%, 10%, and 8% O2, 5 min each) to provide a range of hypoxic stimulation as in our previous studies (38, 39). Baseline parameters were recorded for 15 min before the hypoxic challenge. Initial experiments utilizing two hypoxic challenges revealed that repeated challenges produced similar respiratory responses. Therefore, in subsequent experiments animals were exposed to each oxygen level one time. After the protocol rats were returned to their home cage. One week after surgery (or an equivalent timeframe for controls), this procedure was repeated.
Anesthetized rats.
Rats were anesthetized with isoflurane (3%–5% induction, 2% maintenance, Henry Schein, Dublin, OH) and placed on a Small Animal Physiologic Monitoring System (Harvard Apparatus, Holliston, MA) with ECG leads and temperature probe. Heart rate was recorded with Powerlab data acquisition system (Labchart Version 8, ADInstruments, Colorado Springs, CO). Oxygen flow rate was set at 0.8–1.0 L/min depending on animal size. Baseline measurements were recorded for 15 min with 100% O2. Hypoxic challenge consisted of two exposures (90 s each) of 12% O2 followed by 5 min of 100% O2. Animals were then recovered in their home cage or prepared for surgery. One week after surgery (or an equivalent timeframe for controls), this procedure was repeated with the additional placement of a femoral artery catheter (PE-10 fused to PE-50, A-M systems) for arterial blood pressure monitoring during recording.
Vagal Nerve Transection and Sham Surgery
Rats were anesthetized with isoflurane (3%–5% induction, 1.5%–2.5% maintenance). Temperature was maintained at 38 ± 1°C with a rectal temperature probe and heated platform (Harvard Apparatus). Rats were placed in dorsal recumbency and using aseptic technique, a 1- to 2-cm incision was made in the neck and the right vagus nerve located via blunt dissection. Although somewhat ambiguous in rats (40, 41), the right vagus nerve was chosen because studies in humans and dogs indicate significant cardiorespiratory involvement (42, 43). Vagotomy consisted of the removal of a 1- to 2-mm section of nerve caudal to the nodose ganglion, which contains vagal afferent cell bodies. Sham surgery was identical except the nerve was not sectioned. The incision was closed and rats received buprenorphine (0.01–0.05 mg/kg sc, Par Pharmaceutical, Chestnut Ridge, NY) and 0.9% NaCl (1 mL ip, Hospira Inc., Lake Forest, IL) before recovery. Rats were euthanized 1 wk after surgery. This timepoint was chosen based on studies demonstrating vagotomy-induced neuronal and synaptic alterations in nodose ganglia and nTS neurons within 5–6 days, which persisted 20–30 days (27, 44–46). In vagal afferent nTS tracing experiments, Texas Red-Dextran (TR, ∼500 nL 20% tetramethylrhodamine dextran, Invitrogen, Eugene, OR) was injected into the right nodose ganglion of sham rats as previously described (47). Vagal projections in the nTS were assessed 7 days later.
Rats used for Western blot analysis (n = 18, 6 each group) received identical surgeries except that they were anesthetized with ketamine-xylazine (91/9.1 mg/kg ip, Henry Schein, Dublin, OH), reversed with atipamezole (1 mg/kg sq, Modern Veterinary Therapeutics, Miami, FL), and did not undergo physiological monitoring or hypoxic challenge.
Immunohistochemistry
After the experimental procedures, rats were maintained on isoflurane anesthesia and perfused transcardially with 125-mL Dulbecco’s modified Eagle media (DMEM) followed by 250-mL 4% paraformaldehyde. Brains were removed and the brainstem was separated and sectioned at 30 μm coronally through the nTS on a vibratome and stored in cryoprotectant at −20°C. Immunohistochemistry (IHC) was performed on a 1 in 6 series (i.e., 180 µm separation) from each animal. Brainstem sections containing nTS tissue were washed in phosphate-buffered saline (PBS), pre-blocked with 10% normal donkey serum (NDS) in 0.3% Triton-X 100, washed in PBS, and incubated overnight in 1% NDS with a combination of the following antibodies: guinea pig anti-GFAP (glial fibrillary acidic protein, 1:500, Synaptic Systems 173 004, RRID:AB_10641162), rabbit anti-S100B (S100 calcium-binding protein B, 1:100, Abcam Ab41548, RRID:AB_956280), chicken anti-VGLUT2 (vesicular glutamate transporter 2, 1:500, Synaptic Systems 135 416, RRID:AB_2619824), or guinea pig anti-IBA-1 (ionized calcium-binding adaptor molecule 1, 1:1,000, Synaptic Systems 234 004, RRID:AB_2617105). These antibodies have been verified (WB, IHC) in previously published studies (48–52). One section from each animal was incubated without primary antibodies as a negative control. Sections were then washed in PBS, and incubated for 2 h with appropriate secondary fluorescent antibodies: donkey anti-guinea pig CY5 (1:200, Jackson ImmunoResearch 706-175-148), donkey anti-rabbit CY3 (1:200, Jackson ImmunoResearch 711-165-112), and/or donkey anti-chicken CY2 (1:200, Jackson ImmunoResearch 703-225-115). The sections were then washed in PBS, mounted on gelatin-coated slides, and coverslipped with Prolong Gold (Thermo Fisher Scientific, Eugene, OR).
Brainstem sections were imaged on an Olympus BX50 widefield microscope with a confocal attachment and Neurolucida imaging software (MBF Bioscience, Williston, VT). Single images (×4) were taken as well as 0.5-μm z-step stacks at ×40 and ×60 on the left and right sides at three representative rostral-caudal sections of the nTS that receive high densities of cardiorespiratory inputs (7, 33): nTS at calamus scriptorius (caudal; caudal end of the floor of the fourth ventricle, commissural-medial nTS), mid-area postrema (mid-AP, medial nTS) at 540–720 μm rostral to calamus, and 900–1,080 μm rostral to calamus (rostral, primarily medial nTS).
Western Blot
Vagotomy, sham, and control animals (n = 6 each) were deeply anesthetized with isoflurane and decapitated. Protein extraction and Western blot were performed as previously described (53). The brainstem was rapidly removed, chilled in ice cold N-methyl-d-glucamine-based solution [NMDG, (in mM, pH 7.4, 300 mosM) composed of 137 NMDG, 3 KCl, 1 MgCl2 + 6 H2O, 0.02 CaCl2, 10 glucose, 10 HEPES] and sectioned horizontally on a vibratome. The left and right nTS were separated along the midline, isolated from surrounding tissue, flash frozen in liquid nitrogen and stored at −80°C until use. Tissue was homogenized in buffer containing 250 mM sucrose, 10 mM Tris, 1 mM EDTA, and protease inhibitors (Complete Mini, EDTA-free tablets, Roche), and incubated on ice for 30 min. The homogenate was centrifuged for 10 min at 13,300 rpm, 4°C. The supernatant was collected and protein concentration quantified by the bicinchoninic acid (BCA) method. Protein (15 µg) was mixed with loading dye (Laemmli Sample Buffer, Bio-Rad) and separated on a 4%–20% TGX Gel (Bio-Rad Cat. No. 4561094). The bands were transferred to an Immuno-Blot PVDF membrane (Bio-Rad Cat. No. 1620218). The membrane was preblocked in Tris-buffered saline + 0.1% Tween 20 (TBS-T) with 5% powdered milk for 1 h, rinsed with TBS-T, and incubated with guinea pig anti-GFAP (1:1,000, Synaptic Systems 173 004, RRID:AB_10641162) or the loading control mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:10,000, Chemicon International MAB374, RRID:AB_2107445) in TBS-T/milk overnight at 4°C on a shaker. The membrane was washed with TBS-T and incubated with donkey anti-guinea pig or anti-mouse horseradish peroxidase (HRP, 1:10,000, Jackson ImmunoResearch 706-035-148 or 715-035-151) for 2 h. After being rinsed with TBS-T, the membrane was incubated in Clarity Western ECL (Bio-Rad Cat No. 1705060) for 2 min and imaged on a Bio-Rad Chemidoc. All blots were normalized to GAPDH.
Data Analysis
Cardiorespiratory and immunohistochemical analyses were performed while blinded to the animal identity and group, using Agnosis-FileMask (54).
Cardiorespiratory data for both anesthetized and conscious animals were analyzed in LabChart. To evaluate baseline and hypoxic responses, 5–10 s of data were analyzed from the final minute of each oxygen concentration during breathing in the absence of movement artifacts, sniffing, and augmented breaths. For anesthetized measurements, heart rate (beats per minute) and postsurgery mean arterial pressure (mmHg) were calculated. In conscious animals, respiratory rate (breaths per minute), tidal volume index, and minute ventilation index were calculated. Augmented breaths (sighs) were counted manually from the final three minutes of data for each oxygen concentration in conscious rats (39).
Brainstem images were analyzed with FIJI ImageJ software (55, 56). Glial fibrillary acidic protein (GFAP) is a structural protein used to identify the larger branches of astrocytes (10), whereas ionized calcium-binding adaptor molecule 1 (IBA-1) identified microglia (57). The overall intensity of GFAP and IBA-1 immunoreactivity (-IR) was analyzed in ×4 images inside a 150 × 150 μm box over the left and right nTS or dorsal motor nucleus of the vagus (DMX). Threshold analysis was performed on the image using ×2 the average of the mean grayscale pixel value. The particle analysis function was used to determine the percent area of GFAP or IBA-1.
Astrocyte morphology was analyzed by identifying astrocytes (one per side per animal) in GFAP ×60 image stacks and manually tracing them in three dimensionals (3-D) using the Simple Neurite Tracer plugin (58). The tracings were skeletonized and analyzed using the Analyze Skeleton plug-in and Sholl Analysis, which created concentric spheres at 2-μm intervals from the center of the cell body and determined the number of intersections at each distance (59, 60). Astrocyte cell bodies were identified by immunoreactivity of S100 calcium-binding protein B (S100B), which is found primarily in the cytoplasm and nucleus (61). Astrocyte identity was confirmed by emanating GFAP-IR fibers, and astrocytes were manually counted on ×40 stacks using the Cell Counter plugin. Vesicular glutamate transporter 2 (VGLUT2) identified glutamatergic synapses (62). VGLUT2-IR synapses were counted in the nTS by selecting a 2-µm stack from the center of the VGLUT2 ×40 image stacks and analyzing a 150 × 150-μm area in the center of the image. The image was thresholded, the particle analysis function was used to count overall VGLUT2-IR glutamatergic terminals, and data were normalized to the area. Microglial morphology was analyzed with the FracLac plugin for ImageJ using a modified protocol from Young and Morrison (63, 64). Briefly, Z-projections were generated from ×40 stacks and thresholded. A single microglial cell from the center of the image was isolated and outlined, and the outline was analyzed to generate a bounding area and convex hull. Data such as cell area (number of cell pixels) and cell density (ratio of number of cell pixels to total number of pixels in the convex hull) were obtained.
GFAP and GAPDH bands on Western blot were quantified with the ImageJ Analyze Gels function. GFAP values were normalized to GAPDH for each sample and then standardized to the mean (left and right combined) of control animals. As there were no significant differences between left and right sides, left and right values for each experimental group were averaged and compared among vagotomy, sham, and control.
Statistical Analyses
Prism 8 (Graphpad Software, San Diego, CA) was used for statistical analyses. Normal distribution was assessed with the Shapiro–Wilk test. Data were assessed with ordinary one-way ANOVA, two-way repeated measures ANOVA, or standard two-way ANOVA. Holm–Sidak post hoc analysis was used for all multiple comparisons when appropriate. Heart rate, respiratory rate, tidal volume, minute ventilation, and augmented breath counts during normoxia and various hypoxia levels were compared before and after surgery (or equivalent time period) and among groups. GFAP-IR grayscale and threshold/percent area were compared at three levels of the nTS among control, sham, and vagotomy groups and between intact and manipulated sides of the nTS. IBA-1-IR was analyzed similarly at the mid-AP level of nTS. Sholl and skeleton analyses were used to compare astrocyte branching among experimental groups and between sides. Microglial shape was compared among groups and between sides via FracLac analysis. The number of S100B-labeled astrocytes and VGLUT2-labeled glutamate synapses were compared among experimental groups and between sides. Western blot values were compared between sides and among experimental groups. P values <0.05 were considered significant. Data are shown as means ± SE.
RESULTS
Vagal Afferents Cross Midline and Are Closely Associated with Astrocytes in the nTS
We examined three rostral-caudal levels of the nTS that are involved in cardiorespiratory regulation [relative to calamus scriptorius: caudal (calamus, 0 µm), mid-AP (+ ∼540–720 µm), and rostral (+ ∼1,080 µm)]. Approximate locations are shown in Fig. 1A. We first injected Texas Red-Dextran (TR) into the right nodose ganglion to assess the projection of vagal afferents. Vagal efferent cell bodies in the DMX were visualized ipsilateral to the injection only (Fig. 1B), confirming that vagal efferents do not project contralaterally. However, vagal afferents entering the nTS via the solitary tract (ST) ipsilateral to the injection were observed within both the ipsilateral and contralateral nTS, indicating they cross midline. TR and VGLUT2-IR afferents were present in close proximity to both astrocyte cell bodies (S100B-IR) and their branches (GFAP-IR; Fig. 1, C–G). Similar patterns of organization were seen both ipsilateral and contralateral (not shown) to the TR injection side. In the following analyses, the effect of vagotomy was evaluated in both the manipulated and intact sides.
Figure 1.
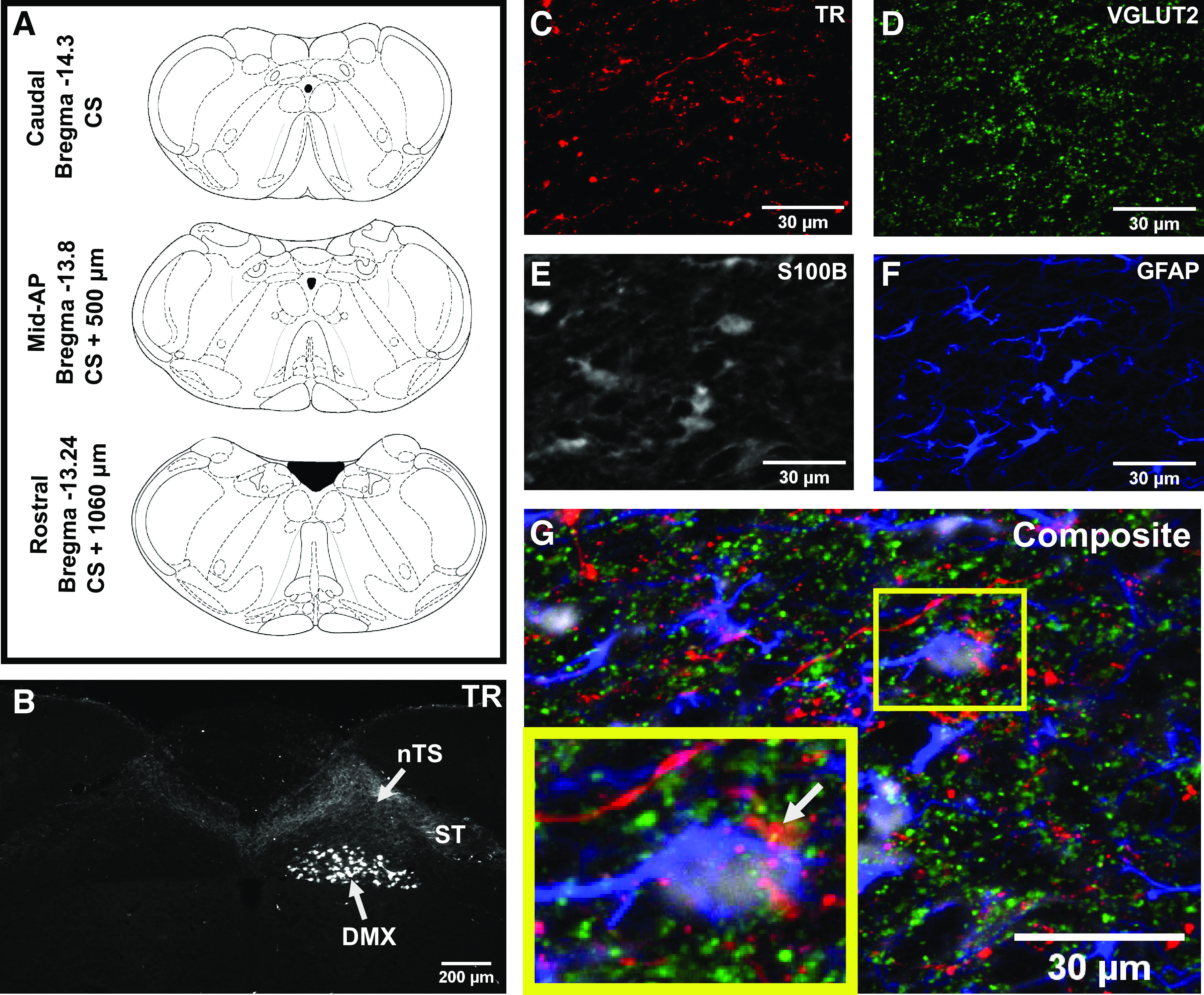
Vagal afferents cross midline in the nucleus tractus solitarii (nTS) and are closely associated with astrocytes. A: coronal brainstem images (65) of approximate locations of caudal, mid-area postrema (AP), and rostral nTS sections evaluated. B: representative image of rat nTS at the level of the AP showing Texas Red Dextran (TR) fluorescence following injection into the right nodose ganglion. Labeled vagal afferents from the right solitary tract (ST) are present in both the right and the left nTS. Dorsal motor nucleus (DMX) neurons are only labeled on the right. Magnified images from the nTS of the section in (B) showing TR-labeled vagal afferents (C), glutamatergic terminals (VGLUT2) (D), and S100B- (E) and glial fibrillary acidic protein (GFAP)-labeled astrocytes (F). G: composite image illustrating their close association. Inset shows a TR-positive glutamatergic terminal (arrow) in close association with an astrocyte cell body.
Vagotomy Increases GFAP Immunoreactivity in the nTS
To assess potential changes in astrocyte expression in the nTS resulting from vagotomy, grayscale images of GFAP immunoreactivity from the three rostral-caudal levels of the nTS from control, sham, and vagotomy animals were obtained. As shown in the representative examples at mid-AP (Fig. 2, A–C, top), we performed threshold analysis within 150 × 150 µm areas of the nTS to compare percent coverage of astrocytic GFAP-IR among groups and between intact and manipulated sides or equivalent sides in controls (control equivalent) (Fig. 2, A–C, bottom). Mean data show that there were no differences in GFAP-IR among rostral-caudal levels of the nTS in sham or control rats (P > 0.05, Fig. 2, D and E). In contrast, on both the intact (Fig. 2D) and manipulated (Fig. 2E) sides of the nTS, unilateral vagotomy increased GFAP-IR percent coverage specifically at the mid-AP level compared with caudal (intact: P = 0.005; manipulated: P = 0.002) and rostral (intact and manipulated: P < 0.001) nTS. Furthermore, on the manipulated side at mid-AP, vagotomy increased GFAP-IR compared with sham (P < 0.001) and control (P < 0.001), and similarly increased GFAP-IR on the intact side compared with sham (P = 0.003) and control (P = 0.002, Fig. 2, D and E). Because vagotomy had the greatest effects at mid-AP, intact and manipulated sides were further compared at this level (Fig. 2F). Unilateral vagotomy increased GFAP-IR to a greater extent on the manipulated side compared with the intact side (P < 0.001), whereas sham and control GFAP-IR were not significantly different between the intact and manipulated sides. Consistent with the increase in GFAP-IR, Western blot analysis demonstrated vagotomy significantly increased GFAP protein (145 ± 22%, n = 6) compared with sham (88 ± 12%, n = 6, P = 0.03) and nearly significantly elevated protein compared with control (100 ± 15%, n = 6, P = 0.08).
Figure 2.

Vagotomy increases astrocytic glial fibrillary acidic protein (GFAP) immunoreactivity in the nucleus tractus solitarii (nTS). Control (A), sham (B), and vagotomy (C) GFAP-immunoreactivity (IR) at mid-area postrema (AP) level of the nTS shown as grayscale (top) and threshold (bottom) images. 150 × 150 µm areas (boxes) in the nTS (outlined in yellow) and the dorsal motor nucleus (DMX) (outlined in green, see Supplemental Fig. S1.) were analyzed in thresholded images. Vagotomy increased GFAP-IR compared with sham and control in both the intact (D) and manipulated (E) (or control equivalent) sides at the mid-AP level of the nTS. In addition, GFAP-IR after vagotomy was greater at mid-AP compared with caudal and rostral nTS on both sides. F: at the mid-AP level of the nTS, vagotomy increased GFAP-IR to a greater degree on the manipulated side. Two-way repeated-measures ANOVA with Holm–Sidak post hoc analysis, Number of rats, control n = 8, sham n = 9, vagotomy n = 9. *P < 0.05.
Vagotomy Increases Astrocyte Branching
Changes in astrocyte structure can contribute to synapse remodeling and alter neuronal function (11). To further characterize the astrocyte response to vagotomy, nTS astrocyte morphology at mid-AP was analyzed in vagotomy, sham, and control animals in the intact and manipulated (or control equivalent) sides via skeletonization and Sholl analysis. Representative astrocyte traces are shown in Fig. 3, A–C and indicate greater branching after vagotomy. Quantitatively, on the intact side (Fig. 3D), there was no interaction between distance from the soma and number of intersections among groups. However, vagotomy had more intersections than control (main effect, P = 0.004). On the manipulated side, vagotomy increased astrocyte branching at several distances from the cell body compared with sham and control. As quantified in Fig. 3E, vagotomy increased the number of intersections compared with sham at 12, 14, and 22 µm (P = 0.02, P = 0.004, P = 0.01) away from the soma, and compared with control at 10, 12, and 14 µm (P = 0.003, P = 0.006, P = 0.02) away from the soma. Analysis of the area under the curve (Fig. 3F) to examine the overall branch intersections demonstrated an increase in vagotomy (main effect) compared with sham (P = 0.009) and control (P < 0.001).
Figure 3.
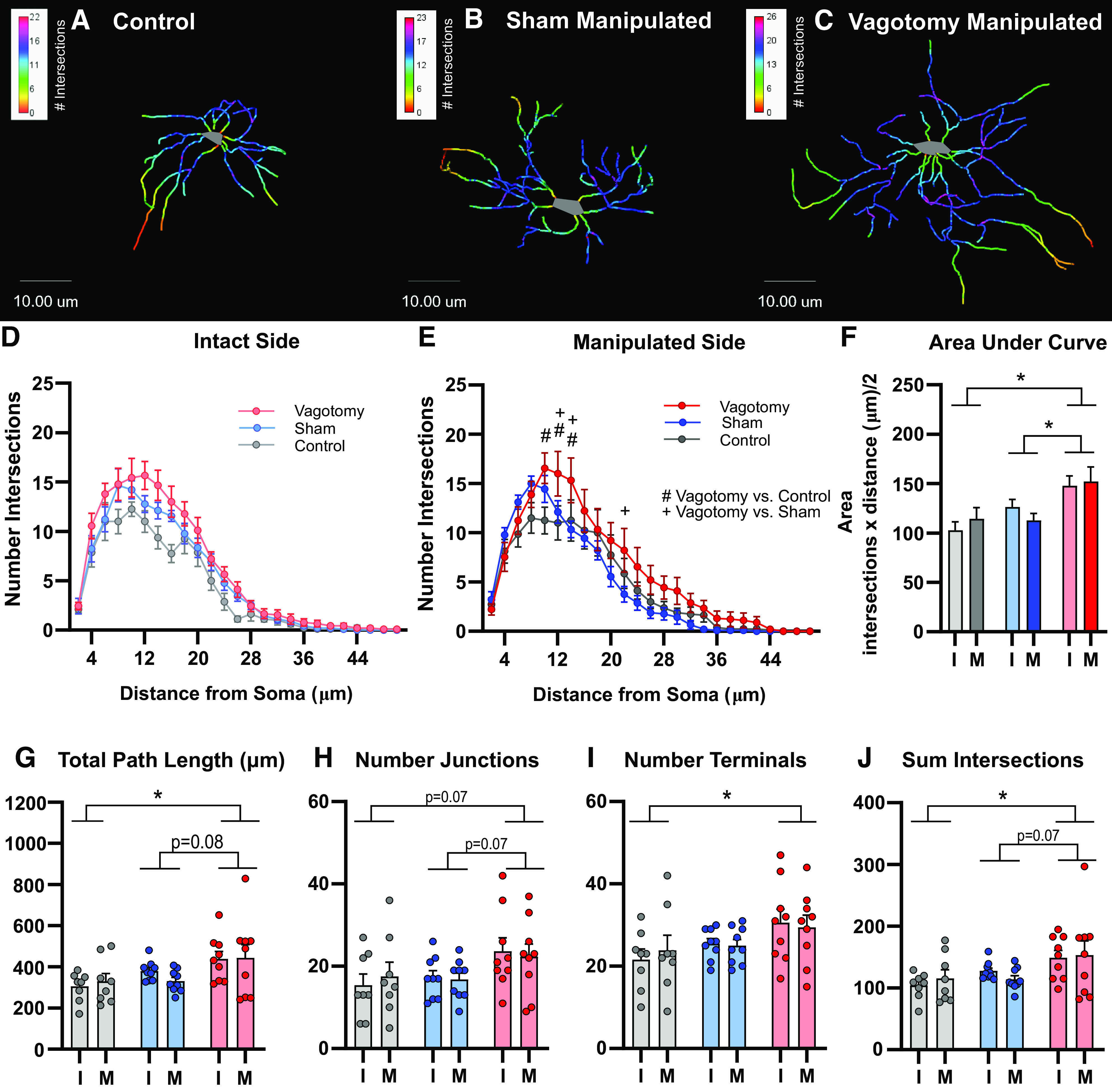
Vagotomy increases astrocyte branching. Representative control (A), sham (B), and vagotomy (C) nucleus tractus solitarii (nTS) astrocyte tracings from the manipulated (or control equivalent) side with Sholl Analysis color overlay. Mean number of intersections at a given distance from the soma as determined by Sholl analysis in intact (D) and manipulated (E) sides for three groups. On the intact side, there was a main effect of vagotomy increasing the number of intersections compared with control. On the manipulated side, vagotomy increased the number of intersections at several distances from the soma: #P < 0.05 for vagotomy vs control, +P < 0.05 for vagotomy vs sham. F: area under the curve (overall branch intersections) for Sholl analysis graphs shown in (D) and (E). Astrocyte total path length (G), number of junctions (H), number of terminals (I), and sum of Sholl intersections (J). Vagotomy increased the astrocyte total path length, number of terminals, and sum of Sholl intersections compared with controls (P < 0.05) and showed a trend toward increasing the total path length (P = 0.077) and number of junctions (P = 0.072) compared with shams. *P < 0.05 main effect between treatment groups. Two-way repeated-measures ANOVA (D, E, G–J), 2-way ANOVA (F), Holm–Sidak post hoc analysis, number of rats, control n = 8, sham n = 9, vagotomy n = 9. I, intact side; M, manipulated (or control equivalent) side.
Further branch pattern analysis revealed that vagotomy increased total path length compared with control (P = 0.02), with a trend compared with shams (P = 0.08, Fig. 3G). There was a trend toward an increased number of junctions in vagotomy compared with both control (P = 0.07) and sham (P = 0.07, Fig. 3H). Vagotomy also increased the number of terminals (endpoints) compared with control (P = 0.04, Fig. 3I). Likewise, vagotomy increased the sum of Sholl intersections compared with control (P = 0.02), with a trend compared with shams (P = 0.07, Fig. 3J). Overall, vagotomy augmented astrocyte branching and trended toward increasing other morphology parameters compared with sham and control.
Vagotomy Does Not Alter the Number of Astrocytes or Glutamatergic Terminals in the nTS
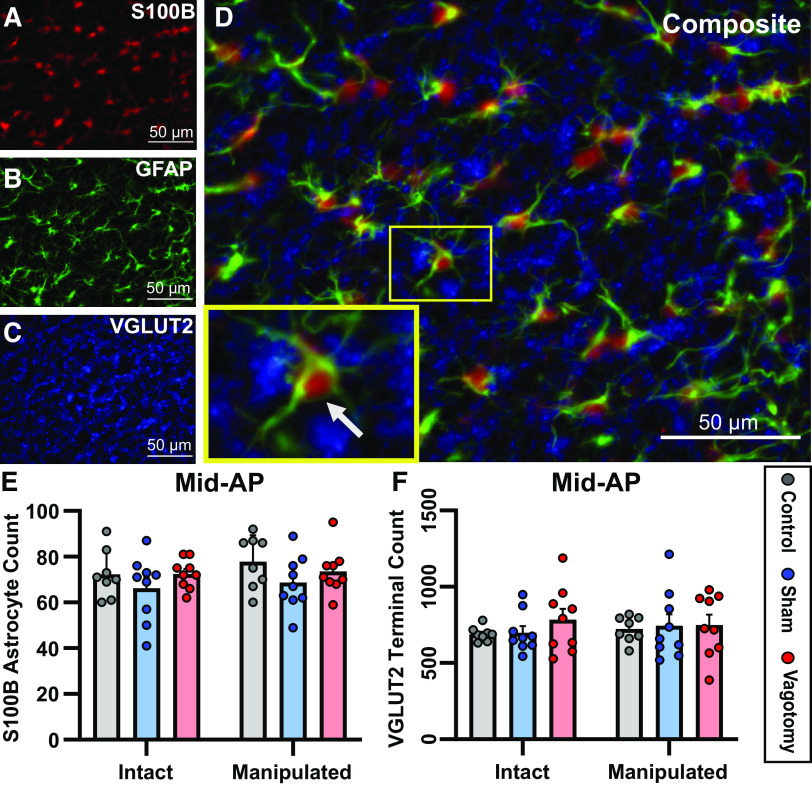
To determine whether the increased GFAP in the nTS associated with vagotomy was due in part to astrocyte proliferation, astrocyte cell counts were performed. Astrocytic cell bodies (S100B) in association with astrocyte cell processes (GFAP) were counted on each side of the nTS at mid-AP (Fig. 4, A, B, and D). There were no differences in number of astrocytes between intact and manipulated (or control equivalent) sides, or among experimental groups (P > 0.05, Fig. 4E).
Figure 4.
Vagotomy does not alter the number of astrocytes or glutamatergic terminals in the nucleus tractus solitarii (nTS) at mid-area postrema (AP). A–C: images showing astrocyte cell bodies (S100B), astrocyte branches (glial fibrillary acidic protein, GFAP), and glutamatergic terminals (VGLUT2) within the nTS. D: composite image, inset showing an astrocyte (arrow) labeled with S100B and GFAP that is surrounded by glutamatergic terminals. Mean number of astrocytes (E) or glutamatergic terminals (F) counted at the mid-AP level of the nTS. There were no differences in the number of astrocytes or the number of glutamatergic terminals among groups or between the intact and manipulated (or control equivalent) side of the nTS. Two-way repeated-measures ANOVA. Number of rats: control n = 8, sham n = 9, vagotomy n = 9.
A previous study found that 10 days after subdiaphragmatic vagal transection, there were fewer afferent terminals in the nTS (66). To determine whether cervical vagotomy alters the overall number of glutamatergic terminals in the nTS, threshold analyses were performed of VGLUT2-labeled glutamatergic terminals at mid-AP (Fig. 4C). There was no effect of vagotomy on the number of nTS glutamatergic terminals compared with shams or controls on either the intact or manipulated sides (P > 0.05, Fig. 4F).
Vagotomy Increases IBA-1 Immunoreactivity and Microglia Numbers, and Alters Microglial Morphology in the mid-AP nTS
Microglia contribute to neuronal and synaptic function and communicate with astrocytes (67, 68). To determine whether microglia also respond to vagotomy, IBA-1-IR was examined at mid-AP, the area with the most significant changes in GFAP-IR. Similar to our astrocyte analysis (see Fig. 2), IBA-1-IR in the nTS at mid-AP was examined in 150 × 150 µm regions of interest using threshold analysis (Fig. 5, A–C). Vagotomy increased the IBA-1-IR percent area compared with sham (P < 0.001) and control (P < 0.001) on the manipulated side (Fig. 5D). In addition, in vagotomized animals IBA-1-IR percent area was greater on the manipulated side compared with the intact side (P < 0.001). To determine if increased IBA-1-IR was related to the number of microglia, counts of IBA-1-positive cells were performed. Vagotomy increased the number of microglia on the manipulated side of the nTS compared with the intact side (P < 0.001) and compared with sham and control on both the manipulated and intact sides (P < 0.001 for all, Fig. 5E).
Figure 5.

Vagotomy increases ionized calcium-binding adaptor molecule 1 (IBA-1) immunoreactivity and microglia numbers, and alters microglial morphology in the mid-area postrema (AP) nucleus tractus solitarii (nTS). Images from control (A), sham (B), and vagotomy (C) rats showing IBA-1-immunoreactivity (IR) at mid-AP as grayscale (top), threshold (middle) and magnified grayscale image as used for cell counting and morphology (bottom). 150 × 150 µm areas (boxes) in the nTS (outlined in yellow) and dorsal motor nucleus (DMX) (outlined in green, see Supplemental Fig. S1) were analyzed in thresholded images. D: mean data at the mid-AP level of the nTS indicate that vagotomy increased IBA-1-IR percent area on the manipulated (or control equivalent) side compared with sham and control and compared with the intact side. E: vagotomy increased the number of microglia counted on both sides compared with sham and control, and increased microglia numbers to a greater extent on the manipulated side compared with the intact side. FracLac analysis of individual microglia showed that vagotomy decreased single cell area (F) and increased cell density (G) compared with sham and control (main effect). Two-way repeated-measures ANOVA with Holm–Sidak post hoc analysis. Number of rats: control/sham/vagotomy: n = 8/6/8 (D), n = 8/8/7 (E), n = 5/5/6 (F and G), *P < 0.05.
Microglia in vagotomized animals were observed to have a more amoeboid appearance (shorter, wider processes) compared with the ramified appearance in sham and control animals (Fig. 5, A–C, bottom). FracLac analysis of individual microglia (63, 64) revealed that vagotomy (main effect) decreased microglial cell area compared with sham (P < 0.001) and control (P < 0.001, Fig. 5F). In addition, vagotomy (main effect) increased density of individual microglial cells compared with sham (P < 0.001) and control (P = 0.01, Fig. 5G).
Vagotomy Increases GFAP and IBA-1 Immunoreactivity in the Dorsal Motor Nucleus of the Vagus
The process of transecting the vagus nerve interrupts not only vagal afferent signaling, but also motor efferent axons whose cell bodies reside in the DMX. To determine if vagotomy causes glial changes in the DMX, GFAP-IR was examined in the caudal, mid-AP, and rostral DMX via thresholding as described for the nTS (see Fig. 2, green box; quantified in Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14394887). On the manipulated side, vagotomy increased GFAP-IR significantly at mid-AP compared with caudal (P < 0.001) or rostral (P < 0.001) DMX (Supplemental Fig. S1A). In addition, at mid-AP GFAP-IR was greater in vagotomy than sham (P < 0.001) or control (P < 0.001). Further examination of the mid-AP level of DMX (Supplemental Fig. S1B) indicated that in vagotomy only, GFAP-IR was greater on the manipulated side compared with the intact side (P < 0.001). Examination of microglia at mid-AP also showed that vagotomy increased IBA-1-IR percent area compared with sham (P < 0.001) and control (P < 0.001) on the manipulated side, with no differences among groups on the intact side (P > 0.05, Supplemental Fig. S1C).
Vagotomy Decreases Augmented Breaths in Hypoxia
We determined whether vagotomy alters baseline cardiorespiratory function and responses to hypoxia in conscious and anesthetized rats. All parameters are shown in Supplemental Tables S1 and S2. In anesthetized animals, there were no differences among groups in baseline heart rate or blood pressure (Supplemental Data Table S1). In addition, vagotomy had no effect on the heart rate or blood pressure response to 12% O2 (one-way ANOVA, P > 0.05, ΔHR: control, 7.7 ± 0.99 beats/min; sham, 10.3 ± 1.45 beats/min; vagotomy, 9.6 ± 2.35 beats/min. ΔMAP: control, −6.3 ± 1.82 mmHg; sham, −6.6 ± 1.11 mmHg; vagotomy, −11.8 ± 4.50 mmHg). In conscious control, sham, and vagotomy animals, baseline respiratory rate, tidal volume, and minute ventilation were similar both before and after surgery (Supplemental Table S1). In addition, the hypoxic ventilatory response was similar among experimental groups before and after surgery (Supplemental Table S2).
Because the vagus nerve influences augmented breath generation in hypoxia, we evaluated augmented breath occurrence in our experimental groups. Figure 6A includes representative raw records showing augmented breaths in a sham and a vagotomy rat during hypoxia (10% O2). Augmented breath counts were not different among groups before surgery (P > 0.05). In sham and control rats, the number of augmented breaths during hypoxia was similar before and after surgery (P > 0.05, Fig. 6, B and C). By contrast, vagotomy decreased the number of augmented breaths in hypoxic conditions at 12% (P = 0.046), 10% (P = 0.003), and 8% (P < 0.001) O2 compared with before surgery (Fig. 6D) and to other experimental groups.
Figure 6.
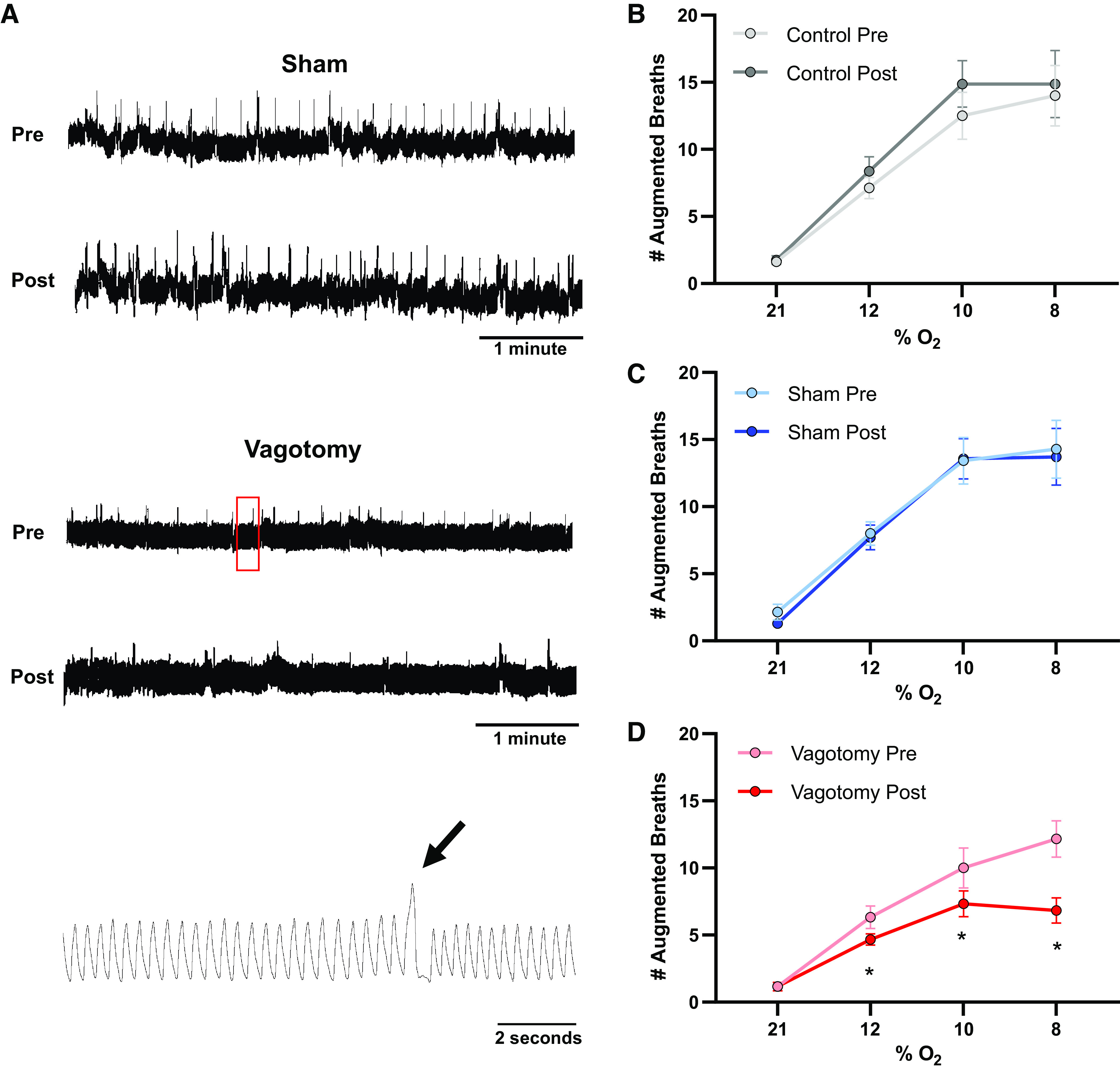
Vagotomy decreases frequency of augmented breaths in hypoxia. A: representative plethysmography traces pre- and postsurgery from a sham (top) and vagotomized (middle) animal breathing 10% O2. Augmented breaths appear as large amplitude deflections in the traces with compressed time scales (∼5 min). The bottom trace shows an expanded time scale of the region in the red box showing an augmented breath (arrow) in the vagotomized rat. Mean data (B–D) indicate that in control (B) and sham (C) animals there were no differences in the number of augmented breaths before and after surgery (or equivalent time period). Vagotomy (D) decreased the frequency of augmented breaths in 12%, 10%, and 8% O2 compared with presurgery counts. Repeated-measures two-way ANOVA with Holm–Sidak post hoc analysis. Number of rats, n = 8 (B), n = 7 (C), n = 6 (D). *P < 0.05.
DISCUSSION
In the present study, we show that unilateral vagotomy alters morphology of nTS glia. Specifically, astrocyte GFAP expression increased within the nTS, primarily at the mid-AP level, with the greatest effects occurring ipsilateral to the transection. Increased GFAP expression occurred due to augmented astrocyte branching rather than astrocyte proliferation. Microglial IBA-1 expression within the mid-AP nTS was also increased and was accompanied by microglial proliferation and process retraction indicative of activation. Similar results were found in the DMX. Functionally, unilateral vagotomy was associated with a reduction in augmented breaths during hypoxia, although baseline breathing and ventilatory responses to hypoxia were not altered. These findings show that altered afferent input (vagotomy) augments astrocyte and microglial expression and morphology and modifies cardiorespiratory output.
The nTS is critical to processing sensory input necessary for cardiorespiratory homeostasis, reflex responses to sensory perturbations, and maintenance of the balance of sympathetic and parasympathetic tone (7, 69). There is substantial evidence that changes in afferent input to the nTS alter neuronal function, both acutely and chronically (5). Such plasticity may promote elevated sympathetic tone, which is associated with morbidity and mortality in many cardiorespiratory diseases (7, 70). Vagal afferents, which provide much of the sensory input to the nTS, are necessary for the development of clinical signs in a mouse model of chronic sympathoexcitation (71). In this study, we cut the vagus nerve distal to the nodose ganglion, leaving afferent cell bodies intact, which has been shown to maintain neuron survival yet decrease excitability presumably due to the lack of sensory input from the viscera (45). Although many studies have focused on acute and long-term effects of prolonged afferent overactivation, such as those that may occur in hypertension or chronic hypoxia, the present study extends our knowledge as to the anatomical and functional plasticity that may occur upon reductions in neural activity such as in spinal cord injury or organ transplant.
Astrocytes ensheath ∼50% of nTS synapses in adulthood where they spatially and temporally limit activation of neuronal neurotransmitter receptors (72). Thus, changes in astrocyte morphology may have substantial functional consequences. In other brain areas, changes in physiological activity or stressors alter astrocyte coverage to permit or limit receptor activation and thus neuronal function (10, 15). GFAP is a major structural protein of astrocytes and is often used as a marker for changes in astrocyte reactivity (astrogliosis), structure, and function (2, 10). Astrogliosis encompasses a range of astrocytic responses based on the severity of insult or injury, and can range from temporary morphological changes to astrocyte proliferation and glial scar formation (15, 73, 74). The increased GFAP we observed following vagotomy likely indicates astrocyte reactivity.
In addition to increased GFAP expression, morphologic changes that occur in astrogliosis include astrocyte hypertrophy, process outgrowth, and altered branching patterns (73). We observed augmented branching and evidence of increased complexity in vagotomized animals, further indicating an astrocyte response to altered nTS input. However, we did not observe an increase in the number of astrocytes. A morphological change in the absence of astrocyte proliferation is characteristic of a milder, indirect insult rather than direct tissue injury (73). The astrocyte changes we observed (increased GFAP and altered morphology) were more similar to nTS astrocyte responses to chronic hypoxia and hypertension (34, 75), and are indicative of altered astrocyte activity in the nTS that may lead to changes in cardiorespiratory function.
Microglia, traditionally considered the resident immune cells of the central nervous system, play a number of other roles including supporting synaptic transmission (20). Microglia also communicate with astrocytes and these glia often rely on signaling from one another to mount reactive responses to stimuli (21). It is not surprising that we found increased numbers of microglia in the nTS in response to vagotomy, given the astrocyte responses, or that the microglia showed morphological evidence of reactivity, including a shift from a ramified to amoeboid appearance (20). Our results confirm previous work demonstrating that subdiaphragmatic vagotomy increases microglia in the nTS (76). Microglia are also involved in nTS synaptic enhancement and elevated blood pressure following sustained hypoxia, and play a role in ventilatory acclimatization to hypoxia (34, 77). Similarly to astrocytes, microglia are activated in the nTS in models of hypertension, heart failure, and obstructive sleep apnea, conditions that likely involve altered afferent input (75, 78–80).
Interestingly, although astrocyte and microglial changes were observed primarily on the ipsilateral side of the nTS, we also observed glial alterations on the contralateral side. This may be due to several mechanisms. For instance, inflammation may be a contributing factor as microglia in the nTS respond to systemic inflammation and glial inflammatory responses have been implicated in many cardiorespiratory diseases (80–82). Alternatively, the presence of both ipsilateral and contralateral nTS changes may be a response to cardiorespiratory effects that occur immediately following vagotomy and resolve over time. Finally, vagal afferents cross the midline and thus the glial responses we observed on the intact side of the nTS may be due to decreased afferent activity on both sides. This explanation is supported by our GFAP and IBA-1 data in the DMX (which does not have contralateral projections) showing only ipsilateral changes in both astrocytes and microglia.
There is ample evidence that astrocytes and microglia respond in a similar manner to changes in sensory input and diseases that do not involve direct neuronal damage and inflammation (34, 73, 75). Neuronal activity influences astrocyte gene expression and metabolism (83) while decreased afferent activity without injury increases GFAP in other brain regions (84, 85). In addition, neuronal activity modulates microglial morphology and motility (86, 87), indicating that both types of glia are responsive to changes in neuronal function. Similarly, previously observed vagotomy-induced neuronal and synaptic changes in the nTS (6–10 days) may be influenced by glial responses which occurred in a similar timeframe in our experiments (27, 66). For instance, vagotomy reduces the amplitude of afferent evoked excitatory postsynaptic currents (EPSCs), increases synaptic failure (27), and decreases spontaneous glutamate release (66). These effects may be the result of changes in glial function such as altered gliotransmitter or glutamate reuptake regulation. For instance, we have shown that block of glutamate re-uptake by astrocyte transporters reduces evoked synaptic currents in nTS and also alters cardiorespiratory responses (8, 14, 88).
The overall number of glutamatergic terminals (evaluated via VGLUT2-IR) in the nTS was not altered in our study, suggesting large-scale synaptic pruning did not occur. Interestingly, reduction in synapses has been observed 10 days after subdiaphragmatic vagotomy (66). Variations in the results of these studies may be due to differences in recovery time, as synapse removal may occur later than 7 days. Differing results may also be due to the methods used to evaluate the presence of synapses, as VGLUT2 used in our study specifically marks glutamatergic synapses, whereas synapses in general were previously examined via synaptophysin. Future studies utilizing electron microscopy are needed to evaluate the potential time-dependent withdrawal and widening of the synaptic cleft as well as changes in the synaptic profile.
In contrast to our initial hypothesis, changes in baseline cardiorespiratory function and chemoreflex responses to hypoxia were not readily observed in anesthetized or conscious animals. Although we predicted tidal volume would increase at baseline and in response to hypoxic challenge after vagotomy due to a blunted Hering–Breuer reflex (89), it was not altered. Relative respiratory rate was also unchanged. The unaltered responses may be due to either compensation by the contralateral intact vagus or other central mechanisms, or lack of convergence or influence of the afferents (e.g., chemoafferents, vagal afferents) responsible for the individual sensory modalities in the nTS. For instance, although there is considerable overlap in the medial (mid-AP) nTS of inputs from arterial baroreceptors, pulmonary stretch receptors, and peripheral chemoreceptors, chemoafferents also send considerable projections to the commissural nTS (69, 90, 91). Thus, while several inputs may converge onto nTS neurons, and astrocytes likely surround these synapses, the astrocytes surrounding chemosensitive nTS neurons may be affected to a lesser degree. Our anatomical evidence supports this notion as vagotomy-induced alterations in astrocytes were less robust in the most caudal nTS compared with those at the medial (mid-AP) region. In addition, while we did not observe significant cardiorespiratory changes 1 wk after vagotomy, these parameters may be altered before or after this time point. Other reflexes more directly dependent on the vagus, for instance the Bainbridge reflex, may be altered by vagotomy and the accompanying glial alteration. Alternatively, changes in glial morphology may be associated with functional compensation, reacting to preserve homeostatic and reflex responses in the presence of abnormal afferent input.
Although evidence of an astrocytic response to vagotomy was apparent, the primary cardiorespiratory change observed was a decrease in augmented breaths induced by hypoxia. Augmented breaths, or sighs, are thought to be a protective reflex functioning to expand atelectatic airways or “reset” the respiratory rhythm (92). Our results support previous studies showing acute bilateral vagotomy eliminates augmented breaths in anesthetized animals (93). Because glia have been shown to influence cardiorespiratory function (2, 23, 80), it is possible that they contribute to the effects of vagotomy on augmented breaths, although the specific role that they play will require additional study.
Perspectives and Significance
In this study, we showed that unilateral vagotomy to decrease afferent input alters astrocyte and microglial morphology in the nTS of the brainstem, a crucial area for cardiorespiratory regulation. This model is not only applicable in terms of direct nerve injury, but is also relevant to the study of cardiorespiratory diseases that affect nTS input. Both astrocytes and microglia exhibited signs of activation/reactivity, clear evidence that functional changes are occurring in the glia themselves, and likely extending to the neurons and synapses that they surround and regulate. Glia have become increasingly recognized as major contributors to neural function in many areas of the central nervous system. Astrocytes and microglia may be key targets for therapeutics not only for neurologic diseases but also for cardiorespiratory diseases whose pathophysiology is associated with changes in the nTS and other areas of the brain. Although this study provides clear evidence of morphologic changes resulting from vagotomy, further investigations are required to determine what specific role glia play in the nTS. Whether such increased branching is associated with increased transporter expression and function or gliotransmitter release is unknown. It is also unclear how these glia interact with nTS neurons, and whether their reactivity interferes with normal function enough to alter neurotransmission, as previous studies using vagotomy might suggest (27, 45, 66). Experiments involving selective glial activation and/or inhibition in the presence of vagotomy will be key to determining the importance of glial responses in nTS function and cardiorespiratory health and disease.
SUPPLEMENTAL DATA
Supplemental material available at: https://doi.org/10.6084/m9.figshare.14394887.
GRANTS
This study was supported by National Institutes of Health HL132836 (E. M. Hasser), HL128454 (D. D. Kline), and 5T32OD011126 (Comp Med).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.C.H., E.M.H., and D.D.K. conceived and designed research; G.C.H. performed experiments; G.C.H. analyzed data; G.C.H., E.M.H., and D.D.K. interpreted results of experiments; G.C.H. prepared figures; G.C.H. drafted manuscript; G.C.H., E.M.H., and D.D.K. edited and revised manuscript; G.C.H., E.M.H., and D.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Heather Dantzler for assistance with immunohistochemistry and Western blot, Colbren Trogstad-Isaacson, Dr. Brian Ruyle, and Allison Naclerio for assistance with plethysmography and data analysis, Sarah Friskey for surgical training, Dr. Diana Martinez and Dr. Ludmila Lima-Silveira for assistance with physiology monitoring and data analysis, and Brandon Nesiba for creating the FileMask program.
REFERENCES
- 1.Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald AJ, Ellacott KLJ. Astrocytes in the nucleus of the solitary tract: Contributions to neural circuits controlling physiology. Physiol Behav 223: 112982, 2020. doi: 10.1016/j.physbeh.2020.112982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruffoli R, Giorgi FS, Pizzanelli C, Murri L, Paparelli A, Fornai F. The chemical neuroanatomy of vagus nerve stimulation. J Chem Neuroanat 42: 288–296, 2011. doi: 10.1016/j.jchemneu.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Chen CY, Horowitz JM, Bonham AC. A presynaptic mechanism contributes to depression of autonomic signal transmission in NTS. Am J Physiol Heart Circ Physiol 277: H1350–H1360, 1999. doi: 10.1152/ajpheart.1999.277.4.H1350. [DOI] [PubMed] [Google Scholar]
- 5.Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir Physiol Neurobiol 164: 105–111, 2008. doi: 10.1016/j.resp.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z, Champagnat J, Poon CS. Phasic and long-term depression in brainstem nucleus tractus solitarius neurons: differing roles of AMPA receptor desensitization. J Neurosci 17: 5349–5356, 1997. doi: 10.1523/jneurosci.17-14-05349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoccal DB, Furuya WI, Bassi M, Colombari DS, Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front Physiol 5: 238, 2014. doi: 10.3389/fphys.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matott MP, Ruyle BC, Hasser EM, Kline DD. Excitatory amino acid transporters tonically restrain nTS synaptic and neuronal activity to modulate cardiorespiratory function. J Neurophysiol 115: 1691–1702, 2016. doi: 10.1152/jn.01054.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDougal DH, Hermann GE, Rogers RC. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J Neurosci 31: 14037–14045, 2011. doi: 10.1523/JNEUROSCI.2855-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 119: 7–35, 2010. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou B, Zuo YX, Jiang RT. Astrocyte morphology: diversity, plasticity, and role in neurological diseases. CNS Neurosci Ther 25: 665–673, 2019. doi: 10.1111/cns.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers RC, McDougal DH, Ritter S, Qualls-Creekmore E, Hermann GE. Response of catecholaminergic neurons in the mouse hindbrain to glucoprivic stimuli is astrocyte dependent. Am J Physiol Regul Integr Comp Physiol 315: R153–R164, 2018. doi: 10.1152/ajpregu.00368.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin LH, Moore SA, Jones SY, McGlashon J, Talman WT. Astrocytes in the rat nucleus tractus solitarii are critical for cardiovascular reflex control. J Neurosci 33: 18608–18617, 2013. doi: 10.1523/JNEUROSCI.3257-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matott MP, Kline DD, Hasser EM. Glial EAAT2 regulation of extracellular nTS glutamate critically controls neuronal activity and cardiorespiratory reflexes. J Physiol 595: 6045–6063, 2017. doi: 10.1113/JP274620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matias I, Morgado J, Gomes FCA. Astrocyte heterogeneity: impact to brain aging and disease. Front Aging Neurosci 11: 59, 2019. doi: 10.3389/fnagi.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theodosis DT, Poulain DA. Neuronal-glial and synaptic plasticity in the adult rat paraventricular nucleus. Brain Res 484: 361–366, 1989. doi: 10.1016/0006-8993(89)90382-x. [DOI] [PubMed] [Google Scholar]
- 17.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci 8: 1078–1086, 2005. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- 18.Oliet SH, Panatier A, Piet R, Mothet JP, Poulain DA, Theodosis DT. Neuron-glia interactions in the rat supraoptic nucleus. Prog Brain Res 170: 109–117, 2008. doi: 10.1016/S0079-6123(08)00410-X. [DOI] [PubMed] [Google Scholar]
- 19.Tadmouri A, Champagnat J, Morin-Surun MP. Activation of microglia and astrocytes in the nucleus tractus solitarius during ventilatory acclimatization to 10% hypoxia in unanesthetized mice. J Neurosci Res 92: 627–633, 2014. doi: 10.1002/jnr.23336. [DOI] [PubMed] [Google Scholar]
- 20.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev 91: 461–553, 2011. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 21.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541: 481–487, 2017. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald AJ, Holmes FE, Beall C, Pickering AE, Ellacott KLJ. Regulation of food intake by astrocytes in the brainstem dorsal vagal complex. Glia 68: 1241–1254, 2020. doi: 10.1002/glia.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez D, Kline DD. The role of astrocytes in the nucleus tractus solitarii in maintaining central control of autonomic function. Am J Physiol Regul Integr Comp Physiol 320: R418–R424, 2021. doi: 10.1152/ajpregu.00254.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Au J, Hawkins T, Venables C, Morritt G, Scott CD, Gascoigne AD, Corris PA, Hilton CJ, Dark JH. Upper gastrointestinal dysmotility in heart-lung transplant recipients. Ann Thorac Surg 55: 94–97, 1993. doi: 10.1016/0003-4975(93)90480-6. [DOI] [PubMed] [Google Scholar]
- 25.Berthoud HR, Shin AC, Zheng H. Obesity surgery and gut-brain communication. Physiol Behav 105: 106–119, 2011. doi: 10.1016/j.physbeh.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colombari E, Talman WT. Denervation supersensitivity to glutamate in the nucleus tractus solitarii after removal of the nodose ganglion. Brain Res 677: 110–116, 1995. doi: 10.1016/0006-8993(95)00131-9. [DOI] [PubMed] [Google Scholar]
- 27.Swartz JB, Weinreich D. Influence of vagotomy on monosynaptic transmission at second-order nucleus tractus solitarius synapses. J Neurophysiol 102: 2846–2855, 2009. doi: 10.1152/jn.00168.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall JM. Chemoreceptors and cardiovascular control in acute and chronic systemic hypoxia. Braz J Med Biol Res 31: 863–888, 1998. doi: 10.1590/s0100-879x1998000700002. [DOI] [PubMed] [Google Scholar]
- 29.Matott MP, Hasser EM, Kline DD. Sustained hypoxia alters nTS glutamatergic signaling and expression and function of excitatory amino acid transporters. Neuroscience 430: 131–140, 2020. doi: 10.1016/j.neuroscience.2020.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda Y, Sato A, Suzuki A, Trzebski A. Autonomic nerve and cardiovascular responses to changing blood oxygen and carbon dioxide levels in the rat. J Auton Nerv Syst 28: 61–74, 1989. doi: 10.1016/0165-1838(89)90008-8. [DOI] [PubMed] [Google Scholar]
- 31.Kollai M, Koizumi K. Reciprocal and non-reciprocal action of the vagal and sympathetic nerves innervating the heart. J Auton Nerv Syst 1: 33–52, 1979. doi: 10.1016/0165-1838(79)90004-3. [DOI] [PubMed] [Google Scholar]
- 32.Siebenmann C, Ryrsø CK, Oberholzer L, Fisher JP, Hilsted LM, Rasmussen P, Secher NH, Lundby C. Hypoxia-induced vagal withdrawal is independent of the hypoxic ventilatory response in men. J Appl Physiol (1985) 126: 124–131, 2019. doi: 10.1152/japplphysiol.00701.2018. [DOI] [PubMed] [Google Scholar]
- 33.Ciriello J, Hochstenbach SL, Roder S. Chapter 2: Central projections of baroreceptor and chemoreceptor afferent fibers in the rat. In: Nucleus of the Solitary Tract, edited by Barraco IRA. Boca Raton, FL: CRC Press, 1994. [Google Scholar]
- 34.Stokes JA, Arbogast TE, Moya EA, Fu Z, Powell FL. Minocycline blocks glial cell activation and ventilatory acclimatization to hypoxia. J Neurophysiol 117: 1625–1635, 2017. doi: 10.1152/jn.00525.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aviles-Reyes RX, Angelo MF, Villarreal A, Rios H, Lazarowski A, Ramos AJ. Intermittent hypoxia during sleep induces reactive gliosis and limited neuronal death in rats: implications for sleep apnea. J Neurochem 112: 854–869, 2010. doi: 10.1111/j.1471-4159.2009.06535.x. [DOI] [PubMed] [Google Scholar]
- 36.Macheda T, Roberts K, Lyons DN, Higgins E, Ritter KJ, Lin AL, Alilain WJ, Bachstetter AD. Chronic intermittent hypoxia induces robust astrogliosis in an alzheimer's disease-relevant mouse model. Neuroscience 398: 55–63, 2019. doi: 10.1016/j.neuroscience.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turlejski T, Humoud I, Desai R, Smith KJ, Marina N. Immunohistochemical evidence of tissue hypoxia and astrogliosis in the rostral ventrolateral medulla of spontaneously hypertensive rats. Brain Res 1650: 178–183, 2016. doi: 10.1016/j.brainres.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruyle BC, Martinez D, Heesch CM, Kline DD, Hasser EM. The PVN enhances cardiorespiratory responses to acute hypoxia via input to the nTS. Am J Physiol Regul Integr Comp Physiol 317: R818–R833, 2019. doi: 10.1152/ajpregu.00135.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King TL, Ruyle BC, Kline DD, Heesch CM, Hasser EM. Catecholaminergic neurons projecting to the paraventricular nucleus of the hypothalamus are essential for cardiorespiratory adjustments to hypoxia. Am J Physiol Regul Integr Comp Physiol 309: R721–R731, 2015. doi: 10.1152/ajpregu.00540.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burkholder T, Chambers M, Hotmire K, Wurster RD, Moody S, Randall WC. Gross and microscopic anatomy of the vagal innervation of the rat heart. Anat Rec 232: 444–452, 1992. doi: 10.1002/ar.1092320313. [DOI] [PubMed] [Google Scholar]
- 41.Stauss HM. Differential hemodynamic and respiratory responses to right and left cervical vagal nerve stimulation in rats. Physiol Rep 5: e13244, 2017. doi: 10.14814/phy2.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giordano F, Zicca A, Barba C, Guerrini R, Genitori L. Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia 58, Suppl 1: 85–90, 2017. doi: 10.1111/epi.13678. [DOI] [PubMed] [Google Scholar]
- 43.Randall WC, Ardell JL, Becker DM. Differential responses accompanying sequential stimulation and ablation of vagal branches to dog heart. Am J Physiol Heart Circ Physiol 249: H133–H140, 1985. doi: 10.1152/ajpheart.1985.249.1.H133. [DOI] [PubMed] [Google Scholar]
- 44.Lancaster E, Oh EJ, Gover T, Weinreich D. Calcium and calcium-activated currents in vagotomized rat primary vagal afferent neurons. J Physiol 540: 543–556, 2002. doi: 10.1113/jphysiol.2001.013121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lancaster E, Oh EJ, Weinreich D. Vagotomy decreases excitability in primary vagal afferent somata. J Neurophysiol 85: 247–253, 2001. doi: 10.1152/jn.2001.85.1.247. [DOI] [PubMed] [Google Scholar]
- 46.Lancaster E, Weinreich D. Sodium currents in vagotomized primary afferent neurones of the rat. J Physiol 536: 445–458, 2001. doi: 10.1111/j.1469-7793.2001.0445c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostrowski TD, Hasser EM, Kline DD.. Depressed GABA and glutamate synaptic signaling by 5-HT1A receptors in the nucleus tractus solitarii and their role in cardiorespiratory function. J Neurophysiol 111: 2493–2504, 2014. doi: 10.1152/jn.00764.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofmann K, Lamberz C, Piotrowitz K, Offermann N, But D, Scheller A, Al-Amoudi A, Kuerschner L. Tanycytes and a differential fatty acid metabolism in the hypothalamus. Glia 65: 231–249, 2017. doi: 10.1002/glia.23088. [DOI] [PubMed] [Google Scholar]
- 49.Hüttenrauch M, Ogorek I, Klafki H, Otto M, Stadelmann C, Weggen S, Wiltfang J, Wirths O. Glycoprotein NMB: a novel Alzheimer's disease associated marker expressed in a subset of activated microglia. Acta Neuropathol Commun 6: 108, 2018. doi: 10.1186/s40478-018-0612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naik AS, Lin JM, Taroc EZM, Katreddi RR, Frias JA, Lemus AA, Sammons MA, Forni PE. Smad4-dependent morphogenic signals control the maturation and axonal targeting of basal vomeronasal sensory neurons to the accessory olfactory bulb. Development 147: dev184036, 2020. doi: 10.1242/dev.184036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ripamonti S, Ambrozkiewicz MC, Guzzi F, Gravati M, Biella G, Bormuth I, Hammer M, Tuffy LP, Sigler A, Kawabe H, Nishimori K, Toselli M, Brose N, Parenti M, Rhee J. Transient oxytocin signaling primes the development and function of excitatory hippocampal neurons. eLife 6: e22466, 2017. doi: 10.7554/eLife.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riuzzi F, Beccafico S, Sagheddu R, Chiappalupi S, Giambanco I, Bereshchenko O, Riccardi C, Sorci G, Donato R. Levels of S100B protein drive the reparative process in acute muscle injury and muscular dystrophy. Sci Rep 7: 12537, 2017. doi: 10.1038/s41598-017-12880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lima-Silveira L, Martinez D, Hasser EM, Kline DD. Mechanisms underlying neuroplasticity in the nucleus tractus solitarii following hindlimb unloading in rats. Neuroscience 449: 214–227, 2020. doi: 10.1016/j.neuroscience.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nesiba B. Agnosis-FileMask. Github, 2019. p. https://github.com/bnesiba/Agnosis-FileMask. [April 28, 2020].
- 55.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18: 529, 2017. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57: 1–9, 1998. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 58.Longair MH, Baker DA, Armstrong JD. Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics 27: 2453–2454, 2011. doi: 10.1093/bioinformatics/btr390. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez-Gonzalez R, Deschamps T, Idica A, Malladi R, Ortiz de Solorzano C. Automatic segmentation of histological structures in mammary gland tissue sections. J Biomed Opt 9: 444–453, 2004. doi: 10.1117/1.1699011. [DOI] [PubMed] [Google Scholar]
- 60.Ferreira TA, Blackman AV, Oyrer J, Jayabal S, Chung AJ, Watt AJ, Sjöström PJ, van Meyel DJ. Neuronal morphometry directly from bitmap images. Nat Methods 11: 982–984, 2014. doi: 10.1038/nmeth.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonçalves CA, Leite MC, Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem 41: 755–763, 2008. doi: 10.1016/j.clinbiochem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Bai L, Xu H, Collins JF, Ghishan FK. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J Biol Chem 276: 36764–36769, 2001. doi: 10.1074/jbc.M104578200. [DOI] [PubMed] [Google Scholar]
- 63.Karperien A. FracLac for ImageJ. 1999–2013. [September 23, 2020]. http://rsb.info.nih.gov/ij/plugins/fraclac/FLHelp/Introduction.htm.
- 64.Young K, Morrison H. Quantifying microglia morphology from photomicrographs of immunohistochemistry prepared tissue using ImageJ. J Vis Exp 136: 57648, 2018. doi: 10.3791/57648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 1998. [Google Scholar]
- 66.Peters JH, Gallaher ZR, Ryu V, Czaja K. Withdrawal and restoration of central vagal afferents within the dorsal vagal complex following subdiaphragmatic vagotomy. J Comp Neurol 521: 3584–3599, 2013. doi: 10.1002/cne.23374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li T, Chen X, Zhang C, Zhang Y, Yao W. An update on reactive astrocytes in chronic pain. J Neuroinflammation 16: 140, 2019. doi: 10.1186/s12974-019-1524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matejuk A, Ransohoff RM. Crosstalk between astrocytes and microglia: an overview. Front Immunol 11: 1416, 2020. doi: 10.3389/fimmu.2020.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colombari E, Sato MA, Cravo SL, Bergamaschi CT, Campos RR, Lopes OU. Role of the medulla oblongata in hypertension. Hypertension 38: 549–554, 2001. doi: 10.1161/01.hyp.38.3.549. [DOI] [PubMed] [Google Scholar]
- 70.Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci 148: 5–15, 2009. doi: 10.1016/j.autneu.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lorenzo-Martín LF, Menacho-Márquez M, Fabbiano S, Al-Massadi O, Abad A, Rodríguez-Fdez S, Sevilla MA, Montero MJ, Diéguez C, Nogueiras R, Bustelo XR. Vagal afferents contribute to sympathoexcitation-driven metabolic dysfunctions. J Endocrinol 240: 483–496, 2019. doi: 10.1530/JOE-18-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chounlamountry K, Kessler JP. The ultrastructure of perisynaptic glia in the nucleus tractus solitarii of the adult rat: comparison between single synapses and multisynaptic arrangements. Glia 59: 655–663, 2011. doi: 10.1002/glia.21135. [DOI] [PubMed] [Google Scholar]
- 73.Schiweck J, Eickholt BJ, Murk K. Important shapeshifter: mechanisms allowing astrocytes to respond to the changing nervous system during development, injury and disease. Front Cell Neurosci 12: 261, 2018. doi: 10.3389/fncel.2018.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32: 638–647, 2009. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melo MR, Gasparini S, Speretta GF, Silva EF, Rodrigues Pedrino G, Menani JV, Zoccal DB, Colombari DSA, Colombari E. Importance of the commissural nucleus of the solitary tract in renovascular hypertension. Hypertens Res 42: 587–597, 2019. doi: 10.1038/s41440-018-0190-6. [DOI] [PubMed] [Google Scholar]
- 76.Gallaher ZR, Ryu V, Herzog T, Ritter RC, Czaja K. Changes in microglial activation within the hindbrain, nodose ganglia, and the spinal cord following subdiaphragmatic vagotomy. Neurosci Lett 513: 31–36, 2012. doi: 10.1016/j.neulet.2012.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lima-Silveira L, Accorsi-Mendonça D, Bonagamba LGH, Almado CEL, da Silva MP, Nedoboy PE, Pilowsky PM, Machado BH. Enhancement of excitatory transmission in NTS neurons projecting to ventral medulla of rats exposed to sustained hypoxia is blunted by minocycline. J Physiol 597: 2903–2923, 2019. doi: 10.1113/JP277532. [DOI] [PubMed] [Google Scholar]
- 78.Daulatzai MA. Pathogenesis of cognitive dysfunction in patients with obstructive sleep apnea: a hypothesis with emphasis on the nucleus tractus solitarius. Sleep Disord 2012: 251096, 2012. doi: 10.1155/2012/251096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ho CY, Lin YT, Chen HH, Ho WY, Sun GC, Hsiao M, Lu PJ, Cheng PW, Tseng CJ. CX3CR1-microglia mediates neuroinflammation and blood pressure regulation in the nucleus tractus solitarii of fructose-induced hypertensive rats. J Neuroinflammation 17: 185, 2020. doi: 10.1186/s12974-020-01857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marina N, Teschemacher AG, Kasparov S, Gourine AV. Glia, sympathetic activity and cardiovascular disease. Exp Physiol 101: 565–576, 2016. doi: 10.1113/EP085713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amorim MR, de Deus JL, Cazuza RA, Mota CMD, da Silva LEV, Borges GS, Batalhão ME, Cárnio EC, Branco LGS. Neuroinflammation in the NTS is associated with changes in cardiovascular reflexes during systemic inflammation. J Neuroinflammation 16: 125, 2019. doi: 10.1186/s12974-019-1512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waki H, Gouraud SS, Maeda M, Paton JF. Evidence of specific inflammatory condition in nucleus tractus solitarii of spontaneously hypertensive rats. Exp Physiol 95: 595–600, 2010. doi: 10.1113/expphysiol.2009.047324. [DOI] [PubMed] [Google Scholar]
- 83.Hasel P, Dando O, Jiwaji Z, Baxter P, Todd AC, Heron S, Márkus NM, McQueen J, Hampton DW, Torvell M, Tiwari SS, McKay S, Eraso-Pichot A, Zorzano A, Masgrau R, Galea E, Chandran S, Wyllie DJA, Simpson TI, Hardingham GE. Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat Commun 8: 15132, 2017. doi: 10.1038/ncomms15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Canady KS, Olavarria JF, Rubel EW. Reduced retinal activity increases GFAP immunoreactivity in rat lateral geniculate nucleus. Brain Res 663: 206–214, 1994. doi: 10.1016/0006-8993(94)91265-3. [DOI] [PubMed] [Google Scholar]
- 85.Canady KS, Rubel EW. Rapid and reversible astrocytic reaction to afferent activity blockade in chick cochlear nucleus. J Neurosci 12: 1001–1009, 1992. doi: 10.1523/jneurosci.12-03-01001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hristovska I, Pascual O. Deciphering resting microglial morphology and process motility from a synaptic prospect. Front Integr Neurosci 9: 73, 2015. doi: 10.3389/fnint.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nebeling FC, Poll S, Schmid LC, Mittag M, Steffen J, Keppler K, Fuhrmann M. Microglia motility depends on neuronal activity and promotes structural plasticity in the hippocampus. bioRxiv 515759, 2019. doi: 10.1101/515759. [DOI]
- 88.Martinez D, Rogers RC, Hasser EM, Hermann GE, Kline DD. Loss of excitatory amino acid transporter restraint following chronic intermittent hypoxia contributes to synaptic alterations in nucleus tractus solitarii. J Neurophysiol 123: 2122–2135, 2020. doi: 10.1152/jn.00766.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moore RL. A study of the Hering-Breuer reflex. J Exp Med 46: 819–837, 1927. doi: 10.1084/jem.46.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res 572: 108–116, 1992. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- 91.Housley GD, Sinclair JD. Localization by kainic acid lesions of neurones transmitting the carotid chemoreceptor stimulus for respiration in rat. J Physiol 406: 99–114, 1988. doi: 10.1113/jphysiol.1988.sp017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Golder FJ, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Augmented breath phase volume and timing relationships in the anesthetized rat. Neurosci Lett 373: 89–93, 2005. doi: 10.1016/j.neulet.2004.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Glogowska M, Richardson PS, Widdicombe JG, Winning AJ. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respir Physiol 16: 179–196, 1972. doi: 10.1016/0034-5687(72)90050-3. [DOI] [PubMed] [Google Scholar]