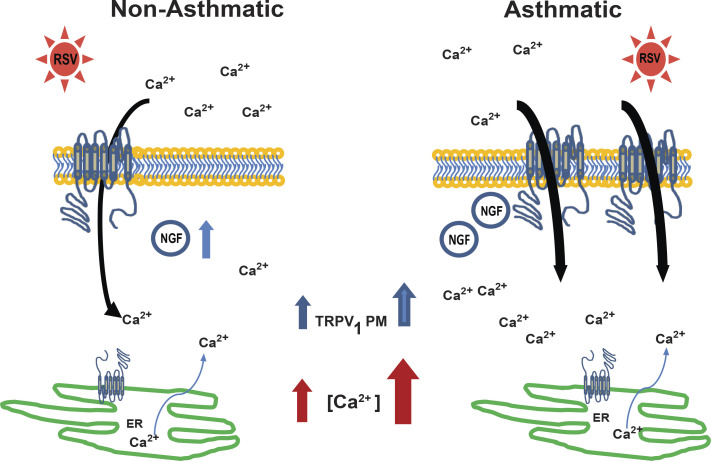
Figure 5.
Modulation of TRPV1-mediated Ca2+ influx in bronchial epithelium. RSV infection of nonasthmatic bronchial epithelial cells induces NGF synthesis. NGF binding to its high-affinity TrkA receptor starts a signaling cascade that leads to TRPV1 expression and its translocation to the plasma membrane, where it can be stimulated by multiple physical or chemical irritants and initiate the inward transfer of Ca2+ from the extracellular compartment to the cytosol. In contrast, basal expression of NGF is already increased at baseline in bronchial epithelial cells from children with asthma, which results in more TRPV1 channels expressed on the plasma membranes and lower threshold of activation. In addition, there is increased expression of intracellular TRPV1 localized to the ER, where it can release Ca2+ from the intracellular storage sites into the cytosol and lead to higher cytosolic Ca2+ concentration at rest. During RSV infection there is a shift of Ca2+ influx from both intracellular stores and extracellular sources to predominantly extracellular sources. Increased intracellular calcium stimulates mucus production, bronchoconstriction, airway barrier permeability, and release of proinflammatory cytokines resulting in the clinical manifestations of airway obstruction in bronchiolitis and asthma. ER, endoplasmic reticulum; NGF, nerve growth factor; RSV, respiratory syncytial virus; TrkA, tropomyosin-related kinase A; TRPV1, transient receptor potential vanilloid 1.