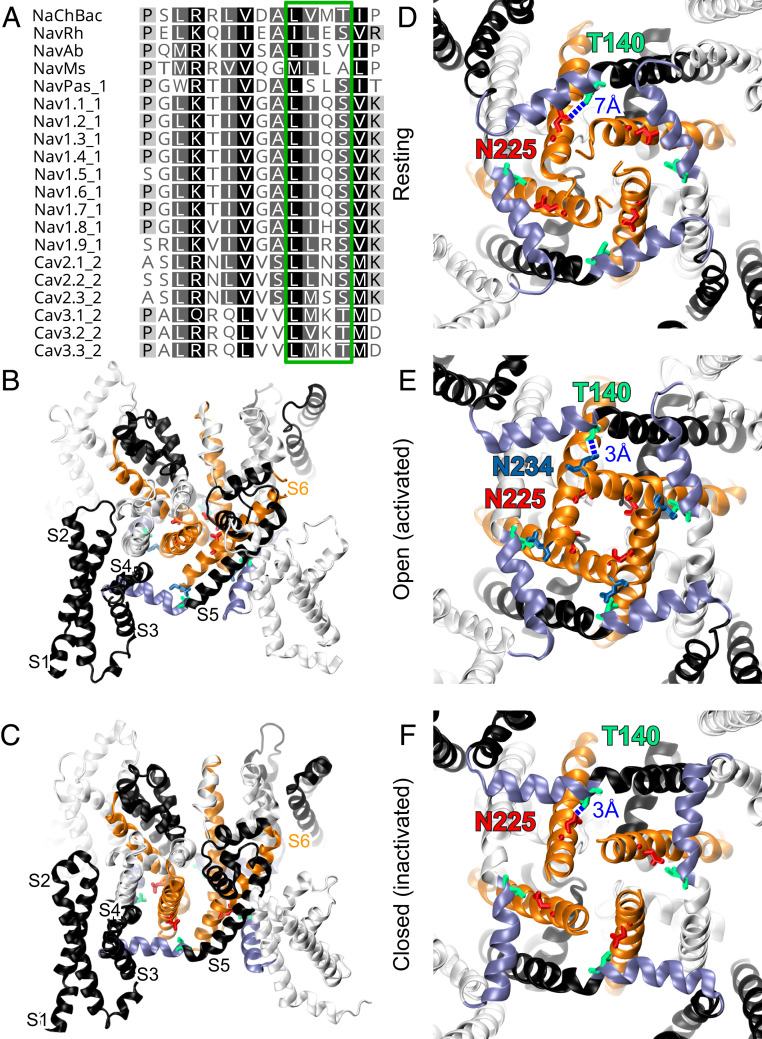
Fig. 1.
Structural models of NaChBac showing pivotal position of T140 in the S4–S5 linker relative to S6 and activation gate. (A) Sequence alignment of S4–S5 linker among NaV and CaV channels. T140 of NaChBac belongs to the LXXS/T motif (green box) at the C-terminal end of the linker, where possible interactions with the S6 helix occur. Overview of aligned (B) open (activated) and (C) closed (inactivated) structural models showing different orientations of S6 helices (orange) between S4–S5 linkers (purple) from the same subunits and the S6 helix of the adjacent subunits. Cytosolic views of NaChBac in (D) resting, (E) open, and (F) closed conformations showing the orientations of the S4–S5 linkers (purple) and S6 helices (orange) in the tetrameric channel. Critical residues T140 (green), N225 (red), and N234 (blue) and their approximate distances are highlighted in blue. Note the hydrogen bonding between the side chains of T140 and N225 in the inactivated state.