FIGURE 4.
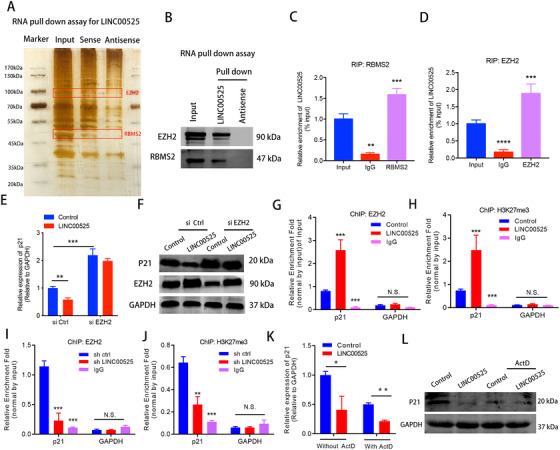
LINC00525 inhibits p21 mRNA transcription in an EZH2‐dependent manner. A. RNA pulldown assay followed by silver staining revealed proteins associated with biotinylated LINC00525. The band corresponding to LINC00525‐interacting proteins were analyzed by mass spectrometry and identified as EZH2 and RBMS2. B. EZH2, and RBMS2 recovered from the LINC00525 pulldown assay were analyzed by western blotting using the indicated antibodies. C‐D. RIP analysis using anti‐RBMS2 (C), and anti‐EZH2 (D) antibodies revealed interaction of the proteins with endogenous LINC00525 in A549 cells. E‐F. EZH2 silencing abrogated the suppressive effects of LINC00525 on p21 mRNA (E), and protein (F) levels, as shown by qRT‐PCR and western blotting. G‐H. ChIP‐qPCR showed that LINC00525 overexpression enhanced the occupancy of EZH2 and its substrate, H3K27me3, on the p21 promoter. IgG was used as the negative control. I‐J. LINC00525 silencing decreased the occupancy of EZH2 and H3K27me3 on the p21 promoter, as shown by ChIP‐qPCR. IgG was used as the negative control. K‐L. The mRNA (K) and protein (L) levels of p21 were significantly reduced in the presence of ActD as determined by qRT‐PCR and western blotting in LINC00525 overexpressing A549 cells. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars, SEM. Abbreviations: RIP, RNA immunoprecipitation; ChIP, chromatin immunoprecipitation; ActD, actinomycin
