FIGURE 5.
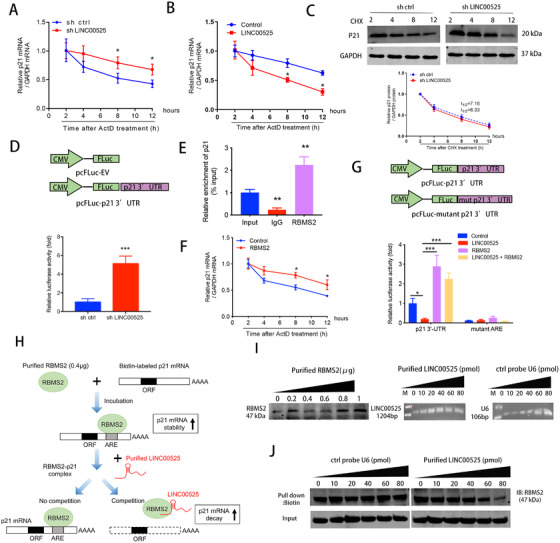
LINC00525 promotes p21 mRNA decay by competitively associating with RBMS2. A‐B. LINC00525 knockdown, LINC00525 overexpressing, and control A549 cells were incubated for the indicated times with 5 μg/mL ActD. RNA was purified and subjected to qRT‐PCR. Knockdown (A) and overexpression (B) of LINC00525 increased and reduced the half‐life of p21 mRNA, respectively. C. LINC00525 knockdown cells were treated with the translation inhibitor, CHX, after which lysates were prepared at the indicated times for western blotting (top). The band intensities were normalized using ImageJ software (bottom). D. Effect of LINC00525 on p21 3'‐UTR reporter activity. A549 cells were transfected with sh LINC00525 prior to pFL‐p21 3'‐UTR transfection, followed by luciferase reporter assay. A549 cells with LINC00525 knockdown showed increased p21 mRNA stability, as indicated by increased p21 3'UTR reporter activity. E. RIP evaluation of the interaction between RBMS2 and p21 3'UTR using an anti‐RBMS2 antibody (5 μg); IgG (5 μg) served as a negative control. F. RBMS2 overexpressing and control A549 cells were incubated with ActD for the indicated times, followed by qRT‐PCR. G. Luciferase reporters containing the p21 3'UTR region and ARE mutant region were constructed. Relative luciferase activity was measured and normalized to Renilla luciferase activity. H. Flow diagram showing the experimental design of purified LINC00525 competitively binding with purified RBMS2 at the 3'UTR of p21 mRNA. I. Amounts of purified RBMS2 protein, purified LINC00525, and control probe U6. J. Different amounts of purified LINC00525 competed with the RBMS2‐P21 complex in a dose dependent manner. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars, SEM. Abbreviations: ORF, open reading frame; 3'UTR, 3'‐untranslated region; ARE, AU‐rich element; CHX, cycloheximide
