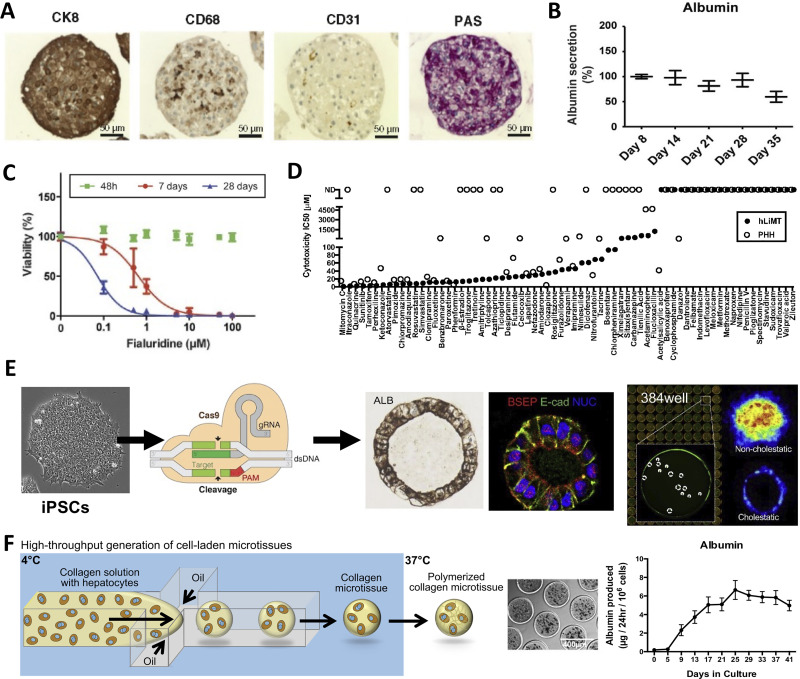
FIG. 3.
3D spheroids, organoids, and bioprinted liver tissues. (a) InSphero spheroids containing primary human hepatocytes (PHHs, stained for glycogen with period acid Schiff, PAS, stain and positive for CK8), liver sinusoidal endothelial cells (CD31 positive), and Kupffer cells (CD68 positive).67 Reprinted with permission from Messner et al., Arch. Toxicol. 87(1), 209–213 (2013). Copyright 2013 Author(s), licensed under a Creative Commons (CC-BY) license. (b) Long-term retention of liver function (albumin shown here) in PHH-containing spheroids generated using commercially available ultra-low attachment plates.70 Reprinted with permission from Bell et al., Sci. Rep. 6, 25187 (2016). Copyright 2016 Author(s), licensed under a Creative Commons Attribution (CC BY) license. (c) Viability assessment via cellular ATP content of fialuridine-induced toxicity was improved over long-term (i.e., 28 days) treatment every 48-h in PHH-containing spheroids generated using ultra-low attachment plates.70 Reprinted with permission from Bell et al., Sci. Rep. 6, 25187 (2016). Copyright 2016 Author(s), licensed under a Creative Commons Attribution (CC BY) license. (d) A panel for drug compounds classified as severe, high, or low clinical drug-induced liver injury (DILI) positive were compared in InSphero PHH-containing spheroids (human liver microtissues or hLiMT) and 2D PHH monocultures; hLiMT predicted more IC50 values than 2D monocultures.73 Reprinted with permission from Proctor et al., Arch. Toxicol. 91(8), 2849–2863 (2017). Copyright 2017 Author(s), licensed under a Creative Commons (CC-BY) license. (e) Left to right: iPSCs, derived from diverse genetic backgrounds can be further gene edited using CRISPR. These iPSCs are then differentiated into foregut cells (not shown) in 2D monolayers, detached, and differentiated into human liver organoids (HLOs) with lumens in 384-well plates.84 High content imaging can be conducted on HLOs to determine the effects of drugs on markers, such as fluorescent bile analog excretion into the HLO lumen. Reprinted with permission from Shinozawa et al., Gastroenterology 160(3), 831–846 (2021). Copyright 2021 Elsevier. (f) A high-throughput droplet microfluidic device for the generation of 3D liver microtissues.85 Left to right: Hepatocytes are suspended in collagen solution and perfused through the microfluidic device at 4 °C (to keep collagen from polymerizing) with an oil stream; since oil and water do not mix, the collagen + cells form spherical droplets. These so-called microtissues are collected, heated at 37 °C to promote collagen polymerization and cell encapsulation, oil is drained, and microtissues are resuspended in culture medium within microwells in static or fluidic plates/devices. The hepatocytes can be cocultured with non-parenchymal cell (NPC) types by either co-encapsulating both cell types within the microtissue or by seeding/coating the NPCs onto the surface of the polymerized collagen-based hepatic microtissues. The PHH microtissues coated with 3T3-J2 fibroblasts display stable liver functions (albumin shown here) for at least 6 weeks in vitro. Adapted with permission from Kukla et al., Gene Expression 20(1), 1 (2020). Copyright 2020 Author(s), licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license.