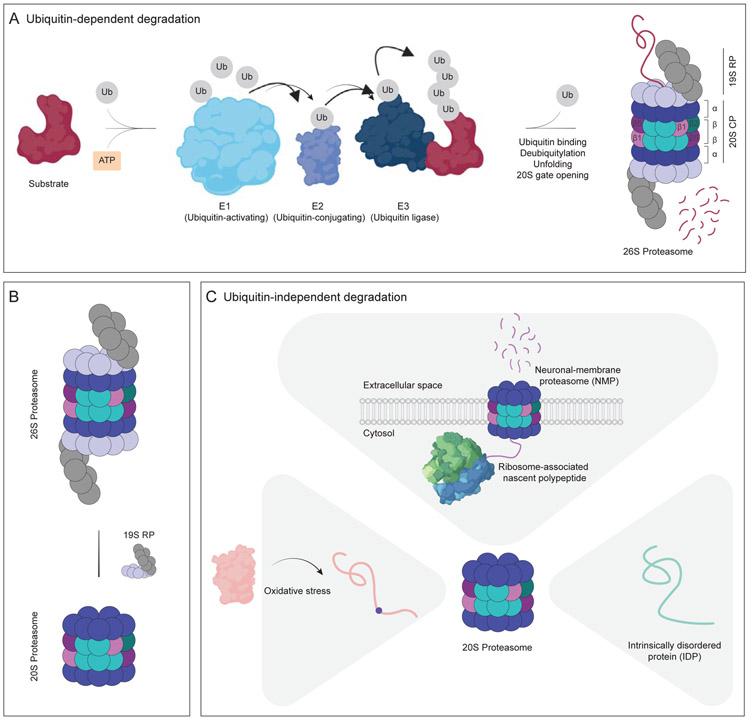
Figure 2. Ubiquitin-dependent and ubiquitin-independent protein degradation by the proteasome.
(A) The ubiquitin-proteasome system (UPS). The ubiquitin-activating enzyme (E1, cyan) hydrolyzes ATP (orange) and binds to ubiquitin (Ub, gray), activating the C-terminus of ubiquitin for nucleophilic attack. Activated ubiquitin is next transferred to the ubiquitin-conjugating enzyme (E2, purple), which then transfers the ubiquitin to the ubiquitin ligase (E3, dark blue). E3 facilitates target selectivity due to its direct binding to the substrate (dark red). As a result of this E1-E2-E3 enzymatic cascade, ubiquitin attaches to the substrate lysine residue via an isopeptide bond. Upon the formation of ubiquitin chains (at least four ubiquitin) on the substrate, it is delivered to the 26S proteasome. The 19S regulatory particle (19S RP) recognizes the ubiquitin chain, deubiquitylates and unfolds the target protein, and opens the 20S gate, driving translocation of the substrate into the 20S catalytic particle (20S CP). (B) The composition of the proteasome is highly dynamic. The 26S proteasome can be decapped under certain circumstances, such as oxidative stress, leading to the accumulation of the 20S proteasome. However, some of the decapped proteasomes can be recapped quickly, leading to an optimal balance of capped and uncapped proteasomes. (C) Ubiquitin-independent degradation by the 20S proteasome. The 20S proteasome, in the absence of any caps, degrades substrates in an ATP- and ubiquitin-independent manner. Proteins modified as a result of oxidative stress (pink) are susceptible to 20S proteasome degradation, potentially due to their exposed hydrophobic patches. Intrinsically-disordered proteins (IDP, teal) comprise the majority of 20S proteasome substrates due to their flexible structure that enables entry into the narrow pore of the 20S catalytic chamber. In neurons, the 20S proteasome localizes to the plasma membrane and degrades ribosome-associated nascent polypeptide chains (purple).