Figure 2. Interactions of P2 group of compound 2 with G48V mutation of PRS17.
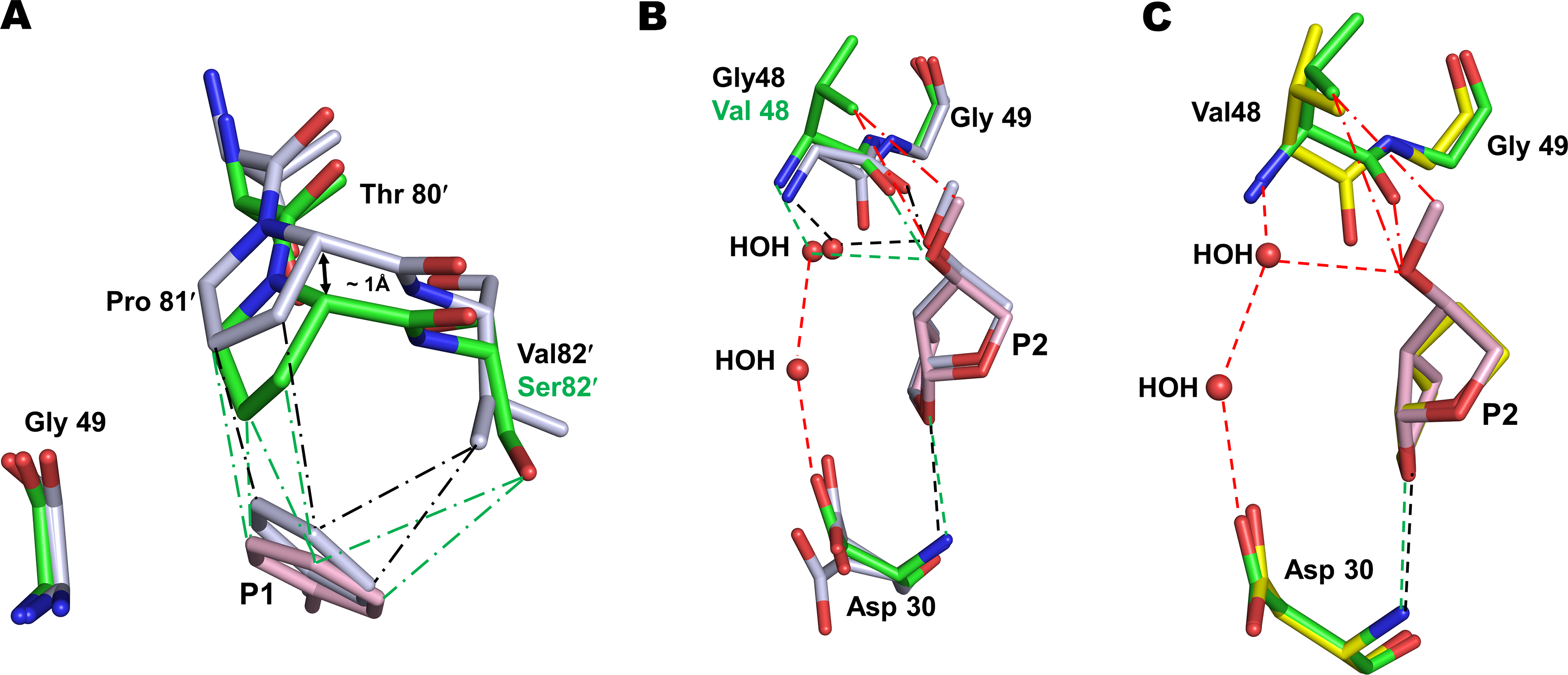
A. The main-chain of residues 80–82 in PRS17/2 complex shifts by ~1 Å due to V82S mutation to maintain van der Waals contacts with P1 group of 2 observed in wild-type PR/2 complex. PR/2 complex is shown as grey sticks colored by element in panels A and B. PRS17/2 amino acids are in green sticks and inhibitor 2 is in pink. Green and black (― - ―) lines represent van der Waals contacts in PRS17/2 and PR/2 complexes, respectively. B. Comparison of P2 methoxy group interaction in the S2 pocket of PRS17/2 and PR/2 complexes. The new interactions of P2 group of 2 are shown in red lines in panels B and C. Green and black (- - -) lines represent hydrogen bonds in mutant and wild-type PR complexes. C. Comparison of interactions at the S2 site of PRS17 by substituted P2 methoxy group of 2 in PRS17/2 complex and bis-THF of 1 in PRS17/1 complex. PRS17/1 is shown as sticks with yellow carbons.
