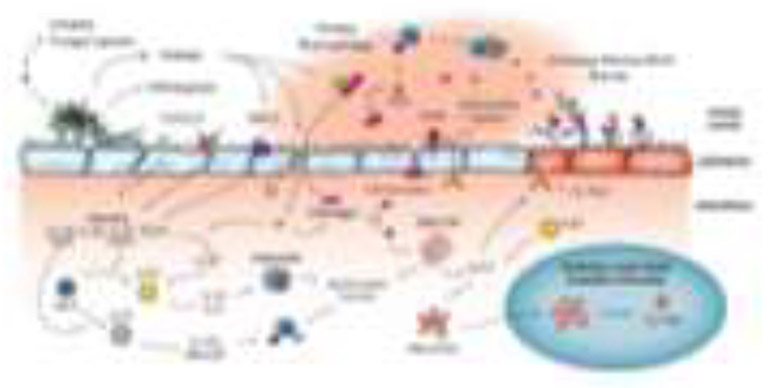
Figure 1.
Recent advances in understanding immune response to airway mycosis. Inhaled fungal spores germinate within the airway and begin releasing immunostimulatory molecules such as chitin, β-glucan, and proteases. Toll-like receptors (TLR) and C-type lectin-like receptors (CLR) recognize antigen on the epithelium to release the alarmins IL-1β, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) that promote adaptive immune responses. Secreted proteases can activate protease activated receptor 2 (PAR-2) on epithelial cells to induce alarmin release, degrade epithelial tight junctions (TJ) to increase permeability and influx of fibrinogen into the airway, and cleave fibrinogen to promote clot formation and the creation of fibrinogen cleavage products (FCP). FCPs are recognized by TLR4 and promote macrophage phagocytic activity, induce mast cell degranulation and IL-13 release, and activate epithelial cells to increase IL-13α1 expression and production of antimicrobial peptides. IL-1β release by epithelial cells promotes progenitor innate lymphoid cells (pILC) development into type 3 ILC (ILC3) to secrete IL-17A and GM-CSF, which enhance macrophage phagocytic and other antifungal activities. ILC2 development from pILC is also induced by alarmins, especially IL-33. Protease-mediate cleavage of full-length IL-33 to a more potent form enhances ILC2 development and cytokine production (IL-13 and IL-5). ILC2 cytokines promote eosinophil recruitment and secretion of antimicrobial peptides, both of which are potently antifungal. ILC2 and FCP-stimulated mast cells released IL-13 that promote goblet cell metaplasia, increase mucus production, and enhanced barrier formation together with fibrinogen. Activated mast cells release IL-13 that activates PD-L2+ dendritic cells (DC) to migrate into regional lymph nodes to again prime adaptive immune responses to both fungi and bystander antigens.