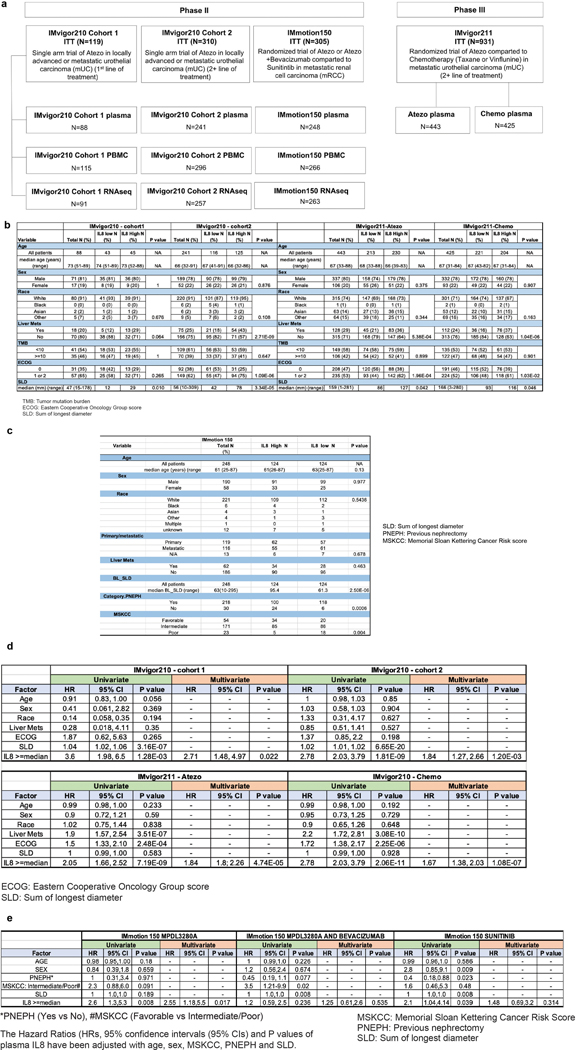
Extended Data Fig. 1. Study profile of IMvigor210, IMvigor211 and IMmotion150 trials.
a, Study profile of IMvigor210, IMvigor211 and IMmotion150 trials. Flowchart showing number of intent-to-treat (ITT) patients IMvigor210, IMvigor211 and IMmotion150, as well as the numbers of patients whose plasma, PBMC and RNAseq samples were included for analysis. Tables showing the demographic characteristics of biomarker-evaluable patients in b, IMvigor210 (n=329) and IMvigor211 (n=868) cohorts, and c, IMmotion150 (n=248) cohort. P values are calculated by two-sided Fisher’s exact test. Tables showing univariate and multivariate logistic regression analyses of baseline plasma IL8 with different factors in overall survival in d, IMvigor210 (n=329) and IMvigor211(n=868) cohorts and e, IMmotion150 (n=248) cohort. HR calculated using stratified Cox proportional hazard regression models, and P values calculated using stratified log-rank test (for details, see Methods). P values were adjusted for multiple comparisons. Multivariate analyses adjusted HRs for age, sex, race, ECOG performance status, presence of liver metastasis, and tumor burden (sum of longest diameter, SLD) in mUC; and age, sex, Memorial Sloan Kettering Cancer Risk (MSKCC) prognostic risk score, previous nephrectomy, and SLD in mRCC data sets.