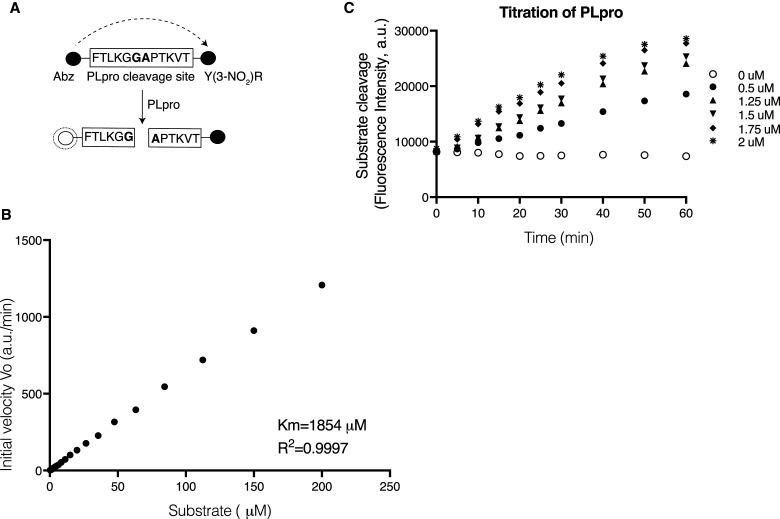
Figure 2. Enzyme assay design and enzyme characteristic.
(A) Schematic of the Pro3 peptide designed for used in the FRET assay. The synthetic peptide contains 12 amino acids from the nsp2/3 junction in the natural polypeptide, in the centre of this is the FTLKGG//APTKVT sequence recognized by PLpro which cleaves between G and A. During synthesis the peptide had the fluorescent Anthranilate (2-aminobenzoyl-Abz) tag added to the N-terminus and the quencher nitro-L-tyrosine (Y(3-NO2)R) fused to the C-terminus. (B) Determination of the enzyme kinetics. The initial velocity of substrate hydrolysis over the titration of substrate is plotted. The velocity did not saturate at the tested concentration, so KM were estimated using Michaelis–Menten equation based on the incomplete dataset. Data was collected from three replicates. (C) Protease activity of PLpro. Titration of the purified enzyme (0.5–2 µM) incubated with the substrate. Fluorescent intensity was measured for 1 h. Data was collected from three replicates.