Abstract
To study the presence of olfactory and gustatory dysfunctions in patients with laboratory-confirmed COVID-19 infection in our set up. Longitudinal study, 1st March 2020-15th August 2020, at a tertiary care hospital. RT PCR positive for SARSCoV-2 patients, above 18 years age included. Excluding patients with previous history of changes in smell or taste sensation, severely ill at the time of admission, history of taking drugs at the time of COVID 19 infection that affect the smell or taste sensation. 435 patients included after obtaining an institutional ethical clearance. After an informed consent, these patients were followed up telephonically, to record any subjective improvement in olfactory or gustatory symptoms and an approximate duration of recovery. Olfactory and/or gustatory dysfunction 10.8% (47/435). Mean (SD) age—34.53(10.8) years. Females affected significantly more [X2 (1, N = 435) = 7.45, p value is 0.006, significant at p < 0.05]. Olfactory dysfunction significantly associated with gustatory dysfunction [X2 (1, n = 435) = 182.29, p < 0.00001]. 19.8% (N = 435) of individuals remained asymptomatic. Nasal symptoms rare (4%, N = 47). Mean (SD) recovery olfactory and gustatory dysfunction 12.1 (7.7) and10.8 (6.3) days respectively. Subjective loss of smell or taste dysfunction was far less common. Women and younger population reported olfactory or gustatory dysfunction commonly. Olfactory and gustatory changes without nasal symptoms, suspicion of COVID-19 infection is relevant. Recovery is complete and early.
Keywords: COVID-19, Olfaction, Gustation, Dysfunction, India, Pandemic
Introduction
A pandemic the scale of COVID-19, has brought the entire world to a standstill, leaving the health care system grappling for tackling this disease. The world has come together in decoding its symptoms, etiopathogenesis and essentially how to tackle it with lesser morbidity. The sheer rapidity of the spread of SARS CoV-2 virus has left gaping holes in its epidemiology.
One such conundrum is the presence of altered smell and taste as prodromal symptom of COVID-19 disease. Following several studies, the presence of olfactory dysfunctions post COVID-19 infection ranged from 5 to 85% in self-reported studies, which was higher than COVID-19-independent post viral olfactory loss. When psychophysical odor identification tests were used, this prevalence ranged from 76% in Europe using the Sniffin’ Sticks (Lechien et al., 2020) to 98% in Iran using the UPSIT (Moein et al., 2020) [1].
Being a cardinal sense, the loss of smell is also known to have significant effect on the quality of life and inability to smell or taste often results in loss of appetite, malnutrition and worsening of general health [2].
Its value, in identifying COVID-19 infection early on, and timely isolation of suspected persons, is extremely crucial for preventing its spread.
Hence the exploration for evidence of occurrence of this important symptom and the course of recovery in a suspected COVID-19 patient is prudent. By this study, we wish to investigate the occurrence and recovery of olfactory and gustatory dysfunction in Indian population.
Material and Methods
Study Design
A longitudinal study was conducted at a tertiary care center. The records of patients were obtained from the hospital Medical Record Section, after obtaining an institutional ethical clearance (IEC/XXXX/XXX/Project/2020–07/CC-01).
Selection and Description of participants
Records of 551 patients who were admitted during the period of 1st March’20 to 30th June’20 at a tertiary care hospital were obtained. Amongst that, 435 patients who fit the eligibility criteria of age above 18, diagnosed with COVID-19 infection via RT-PCR in oropharyngeal/nasopharyngeal swab were considered for the study. The patients having previous history of changes in smell or taste sensation (due to other health conditions—like nasal polyp, nasal surgery or trauma, head trauma, liver disease, hypothyroidism), or were severely ill at the time of admission hence, were unable to give detailed history and patients with history of taking drugs, at the time of COVID 19 infection, that commonly affect the smell or taste sensation (nifedipine, phenothiazines, decongestant spray, chemotherapy etc.) were excluded.
Evaluation
Socio-demographic data, detailed history and examination findings were noted from the records in a SELF MADE CASE PROFORMA (Fig. 1). A note was made, if the records stated a complaint of change in smell (hyposmia—decreased smell/anosmia—complete loss of smell) and taste (hypogeusia—decreased taste/ ageusia—complete loss of taste) immediately before or after the diagnosis of COVID 19 (the timing of onset). The necessity of Intensive Care Unit or discharge without its requirement was noted from the records as a measure of clinical outcome (discharged in stable conditions with or without ICU admission).
Fig. 1.
Case proforma
After an informed consent from all patients, these patients were followed up telephonically, at a minimum duration of 6 weeks after discharge and a maximum of 4 months to record any subjective improvement in olfactory or gustatory symptoms and an approximate duration of recovery.
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Statistical Analysis
Data entry was done in MS Excel software. Statistical Package for the Social Sciences for Windows (SPSS version 21.0) was used to perform the statistical analyses. The potential associations between epidemiological, clinical and olfactory and gustatory outcomes have been assessed through cross-tab generation between two variables (binary or categorical variables) and Chi-square test. Incomplete responses were excluded from analysis. A level of p < 0.05 was used to determine statistical significance.
Results
We studied the records of 435 patients laboratory confirmed COVID 19 positive patients. The age ranged from 18 to 82 [mean (SD)—38.25(13.95)] and a gender distribution of male to female ratio of 2:1 was observed. The study showed the most common symptom as fever (59.8%) followed by cough (44.4%). The most common ENT symptom was sore throat (19%). In general the study observed no symptoms at all in 1/5th (19.8%, N = 435) of individuals with positive COVID-19 status. They continued to be asymptomatic and were discharged following RT- PCR negative for COVID 19.
Olfactory and Gustatory Dysfunction Epidemiology
The presence of olfactory and/or gustatory dysfunction was seen in 10.8% (47/435) of the studied population. The mean (SD) age of the population affected with olfactory and/or gustatory symptoms was 34.53(10.82) years with the most common age group affected as 20–29 years old. A chi square test of independence was performed to examine the relation between gender and the presence of olfactory and gustatory dysfunction. Females were likely to be affected more with olfactory and gustatory dysfunction. [X2 (1, N = 435) = 7.45, p value is 0.006, significant at p < 0.05].
The distribution of the presence of olfactory with gustatory dysfunction was noticed in 24 (51%, N = 47), only olfactory changes in 11 (23%, N = 47) and only gustatory changes in 12(26%, N = 47) (Fig. 2). Our study showed, that olfactory dysfunction was significantly associated with gustatory dysfunction [X2 (1, n = 435) = 182.29, p < 0.00001].
Fig. 2.
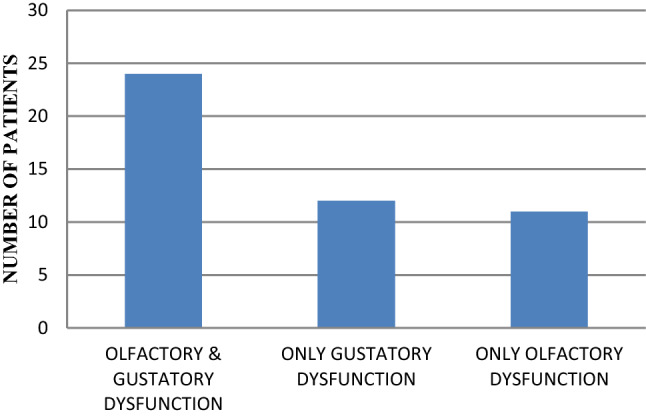
Presence of olfactory and/or gustatory dysfunction
Out of the 35 patients with olfactory dysfunction, 28 (80%) complained of complete loss of smell (Anosmia) subjectively while the remaining 7 (20%) reported subjectively decreased sense of smell (hyposmia).
Gustatory outcomes showed a subjective complete loss of taste (Ageusia) in 13 patients out of 36 patients (36%) with gustatory complaints and subjective hypogeusia (decreased sense of taste) in the rest of the 64% patients.
Amongst the non ENT symptoms, Fever and cough were the most associated symptoms in patients with olfactory and gustatory symptoms (64%, 55%, N = 47). Sore throat was the most common ENT symptom associated with olfactory and gustatory dysfunction. Isolated olfactory dysfunction in otherwise asymptomatic COVID-19 positive patients, was observed in only 4 patients only. Nasal symptoms were noticed in 4.8% of the total population studied (20, N = 435), with only 2 patients reporting accompanying olfactory or gustatory dysfunctions (Fig. 3).
Fig. 3.
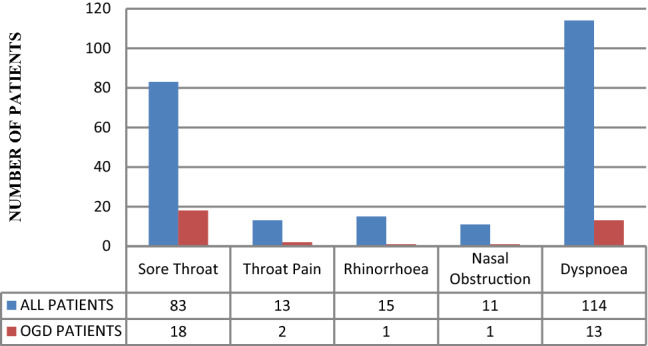
Otorhinolaryngologial symptoms
Recovery
The mean (SD) recovery for olfactory dysfunction was 12.1 (7.7) days. It was noticed that most patients started recovering on the 7th day after the olfactory dysfunctions started, and completely recovered by the 14th day (Fig. 4).
Fig. 4.
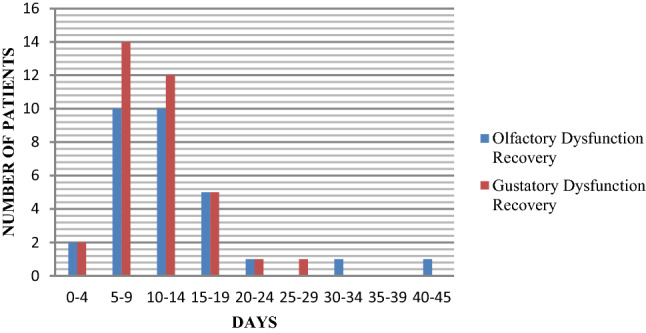
Recovery time
The mean (SD) recovery for gustation was seen by 10.8 (6.3) days. It was noticed that most patients started recovering on the 7th day after the gustatory dysfunctions started, and completely recovered by the 14th day (Fig. 4).
On 6 week follow up, 3 patients with only olfactory dysfunctions, reported an incomplete recovery. Two patients with olfactory and gustatory dysfunction reported incomplete recovery of olfactory dysfunction whereas an incomplete recovery of gustation in only 1.
Clinical Outcome
A chi-square test of independence was performed to examine the relation between olfactory and gustatory dysfunction and the clinical outcome of COVID-19 positive patients (requirement of ICU or discharged without any requirement of ICU). The relation between these variables was not significant, [X2 (1, N = 435) = 0.033, p = 0.855841, Not significant at p < 0.05].
Hence, our study did not show better clinical outcomes in patients with OGD (olfactory or gustatory dysfunction) when compared to patients without it.
Healthcare Workers (HCW)
A chi-square test of independence was performed to examine the relation between olfactory and gustatory dysfunction in healthcare workers with COVID-19 infection. The relation between these variables was not significant at p < 0.05 [X2 (1, N = 435) = 2.19, p = 0.14].
But a relative risk of 1.50 (95% CI = 0.88–2.58, p = 0.13) was noticed in HCW for presence of olfactory and gustatory dysfunction.
Also, it was noticed that 4 patients who did not notice any recovery in olfactory dysfunction at 6 weeks follow up, were all Health care workers.
Discussion
Olfaction and gustation profoundly impacts humanity and its loss is deeply disturbing to all. Concerns regarding new onset smell and taste dysfunctions, as a marker for identification of COVID-19 infection, has been fuelled by multiple studies in the past few months. However, a great variability has been found (from 5 to 95%) in the incidence of loss of smell that probably depends on the methodology of questionnaires and the lack of quantitative assessment given the circumstances.
A retrospective analysis of the records of 435 laboratory confirmed COVID-19 patients admitted at our hospital over a period of 4 months, elicited an occurrence of subjective loss of smell and/or taste in only 47 patients (10.8%). This is glaringly in contrast to studies conducted for various western populations [3].
These population differences may be explained by two different scenarios: at the level of the virus or host. A virus mutation (D614G) may be causing differing infectivity. While at the level of the host, different frequencies of genetic variants of the SARS-COV-2 virus entry proteins may be present in the olfactory epithelium and taste buds which may lead to differential susceptibility to chemosensory dysfunctions. It is likely that both, virus and host factors, contribute to the variation in the prevalence of chemosensory dysfunction. Both explanations have major implications for infectivity, diagnosis, and management of the COVID-19 pandemic [4].
It has been established via several studies that SARS-CoV-2 uses the human angiotensin -converting enzyme 2 (hACE2) as the receptor for host cell entry mainly through endocytosis and uses the Transmembrane Serine Protease 2 (TMPRSS2) for S protein priming along with activation [5, 6].
Srivastava et al. [7], further showed a difference in the allele frequency amongst Europeans and Asians for a polymorphism rs2285666, present in ACE2. For the first time, they ascertained a significant positive correlation for alternate allele (T or A) of rs2285666, with the lower infection as well as case-fatality rate among Indian populations.
Hereby, reiterating that the COVID-19 virus and host receptor protein variability results in varying presentation amongst human beings.
Our study showed a significant association between the presence of olfactory and gustatory dysfunctions, fueling the idea that gustatory dysfunction was primarily because of affliction of retronasal pathway of gestation [5, 8].
However, some studies reported high ACE2 expression on the oral cavity mucosa and the epithelial cells of the tongue. The presence of few patients with only gustatory changes in our study suggests another possibility, that SARS-CoV-2 may have an effect on the taste buds or receptors directly [5, 9, 10].
Predominantly, the age group affected was younger (20–29 years) [2]. The possible reason for this could be unawareness of olfactory dysfunction amongst middle and old age patients [5, 11], along with a less likely tendency to report [4].
We observed that females were likely to be affected more with olfactory and gustatory dysfunction. Women are more likely to experience emotional issues such as depression, anxiety related to olfactory impairment [5, 12].
Various studies like Chary et al. (2020) [13], reported olfactory impairments were more frequently reported in the female population, young people, and house-bound patients with mild symptomatic forms.
Isolated olfactory or gustatory dysfunction was not a norm amongst the studied population and this has been supported by other studies like Kosugi et al. [14] and Hopkins et al. [15]. This could be because of lack of awareness amongst the people about this seemingly trivial symptom during the initial period of COVID-19 pandemic.
Amongst COVID-19 infected patients studied, nasal symptoms were negligible in patients who reported olfactory or gustatory changes. Even though the affected group in our study is small, it would be wise to consider the presence of sudden onset olfactory or gustatory dysfunctions without nasal symptoms as an indicator to testing for a COVID-19 infection [3, 13–18].
It is worth a mention that a higher number of patients reported subjective anosmia (80%) while subjective hypogeusia was more prevalent (64%) amongst the population with chemosensory deficit. Unfortunately a lack of quantitative evaluation of chemosensory deficits, prevents us from understanding why olfaction is more severely affected.
Despite being a perturbing symptom, a short recovery time is observed in most of our patients for both olfaction and gustation, a finding similar to other studies [3]. Incomplete recovery was observed in 10.6% (N = 47) of the population affected with olfactory and gustatory symptoms, when followed up. (Affection of supportive cells rather than the neuroepithelial cells [5]).
At present, short term smell and taste recovery rate is approximately 44–74%, which is higher than previous reports of other post-viral olfactory dysfunction such as rhinovirus, influenza, respiratory syncytial virus, and other coronaviruses. Nevertheless, it is still too early to assess long term olfactory improvement in COVID-19 patients, as well as gustatory improvement [13, 14, 19].
We did not observe better clinical outcomes in patients with olfactory or gustatory dysfunction when compared to patients without the dysfunction. Hopkins et al. [20] although unable to attribute any prognostic significance to the presence of anosmia without widespread epidemiologic study across large populations, did observe 2 different phenotypes of COVID-19 disease; those with mild disease where anosmia is prevalent, found more often in younger and female patients, and those with moderate to severe respiratory disease, seen more frequently in older and male patients. It might be that smell and taste disturbance simply goes unreported in the presence of respiratory distress. Long-term studies of COVID-19 survivors are required, as well research to determine for how long patients may remain infectious.
We also observed a relative risk of 1.55 times in HCW for presence of olfactory and gustatory dysfunction when compared to non-health care workers. It is interesting to note that all 5 patients who did not show subjective recovery in olfactory gustatory symptoms at 6 weeks were noted to be healthcare workers. Since the number of patients with subjective olfactory or gustatory dysfunction in our study is small, hence we cannot comment if the healthcare workers are at greater risk or have a severe form of this dysfunction.
Work by Hunter and colleagues [21, 22], observes a comparable rate of COVID-19 positivity in frontline clinical staff compared with non-clinical staff in hospitals. The authors suggest this shows isolation and PPE measures are adequate at present to prevent nosocomial infections and the transmission may reflect that from the community.
A study to further the understand disease progression in Health care workers, is a necessary step forward, as the nature of such professions means higher likelihood both of contracting the virus and of spreading the virus if they were to catch it and anosmia may be an early symptom of this [21].
We feel the strength of our study lies in the number of patients over a time period having similar exposures. It is also one of the few studies conducted in this region. It being a nation affected in large numbers, our study answers several questions related to epidemiology of the disease. Being a retrospective longitudinal study it is subject to recall bias. A smaller number of patients were observed to have olfactory gustatory dysfunction. We were unable to quantify the olfactory and gustatory dysfunction. We were unable to associate negative RT-PCR with Olfactory and gustatory dysfunction recovery. This study sets a path for further prospective studies regarding the behavior of this disease.
Conclusion
In our study, subjective observation of loss of smell or taste dysfunction was far less common. Women and the younger population reported olfactory or gustatory dysfunction commonly. In a set up of olfactory and gustatory changes without nasal symptoms, suspicion of COVID-19 infection is relevant. Recovery in most cases is complete and early. Health care workers were noticed to be at slightly more risk than the rest.
Authors' Contributions
PL: The lead author has conceptualized and been a key author for the formation of the entire study, including the analysis of raw data and its implications. PC: The corresponding author, is responsible for the collection of data, analysis and final implications of the results. She along with PL have reviewed many articles related to this study and reached the conclusions stated in the article. IPT: She is responsible for reviewing the data, results, proof reading of the article. SJ is responsible for the collection of data. She along with PL and PC have reviewed many articles related to this study and reached the conclusions stated in the article. SSN: Responsible for collection of data. SSh reviewed articles for formation of major part of the discussion. ST: proof reading of the article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of Data and Material
Available.
Declarations
Ethics Approval
The study was approved by the Institutional ethical committee IEC/XXXX/XXX/Project/2020–07/CC-01.
Consent to Participate
An informed consent was procured telephonically from all the participants for participation. No data which identifies a patient was used.
Consent for Publication
An informed consent was procured telephonically from all the participants for publishing of their data. No data which identifies a patient was used.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Priti Lal, Email: drpritilal@yahoo.com.
Priyanka Chamoli, Email: priyankachamoli88@gmail.com.
Isha Preet Tuli, Email: ishatuli@gmail.com.
Shweta Jaitly, Email: shweta.vmmc@gmail.com.
S. N. Sneha, Email: sneha.nagendra94@gmail.com
Shilpam Sharma, Email: shilpam30@gmail.com.
Sandeep Trehan, Email: sandytrehan89@gmail.com.
References
- 1.Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, et al. More than smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 2020;45(7):609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullol J, Alobid I, Mariño-Sánchez F, Izquierdo-Dominguez A, Marin C, Klimek L, et al. The loss of smell and taste in the COVID-19 outbreak: a tale of many countries. Curr Allergy Asthma Rep. 2020;20(10):61. doi: 10.1007/s11882-020-00961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277(8):2251–2261. 10.1007/s00405-020-05965-1. Epub 2020 Apr 6. (PMID: 32253535; PMCID: PMC7134551) [DOI] [PMC free article] [PubMed]
- 4.von Bartheld CS, Hagen MM, Butowt R (2020) Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem Neurosci 11(19):2944–2961. 10.1021/acschemneuro.0c00460. Epub 2020 Sep 17. (PMID: 32870641; PMCID: PMC7571048) [DOI] [PMC free article] [PubMed]
- 5.Kanjanaumporn J, Aeumjaturapat S, Snidvongs K, Seresirikachorn K, Chusakul S (2020) Smell and taste dysfunction in patients with SARS-CoV-2 infection: a review of epidemiology, pathogenesis, prognosis, and treatment options. Asian Pac J Allergy Immunol 38(2):69–77. 10.12932/AP-030520-0826. (PMID: 32563234) [DOI] [PubMed]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S et al (2020) SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280.e8. 10.1016/j.cell.2020.02.052. Epub 2020 Mar 5. (PMID: 32142651; PMCID: PMC7102627) [DOI] [PMC free article] [PubMed]
- 7.Srivastava A, Bandopadhyay A, Das D, Pandey RK, Singh V, Khanam N, et al. Genetic Association of ACE2 rs2285666 polymorphism with COVID-19 spatial distribution in India. Front Genet. 2020;11:564741. doi: 10.3389/fgene.2020.564741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54(26):1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaira LA, Salzano G, Fois AG, Piombino P, De Riu G (2020) Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol 10(9):1103–1104. 10.1002/alr.22593. Epub 2020 Jun 15. (PMID: 32342636; PMCID: PMC7267531) [DOI] [PMC free article] [PubMed]
- 11.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL (2020) Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol 10(8):944–950. 10.1002/alr.22587. Epub 2020 Jun 18. (PMID: 32301284; PMCID: PMC7262123) [DOI] [PMC free article] [PubMed]
- 12.Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schöpf V, Mainland JD, et al. Anosmia—a clinical review. Chem Senses. 2017;42(7):513–523. doi: 10.1093/chemse/bjx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chary E, Carsuzaa F, Trijolet JP, Capitaine AL, Roncato-Saberan M, Fouet K et al (2020) Prevalence and recovery from olfactory and gustatory dysfunctions in Covid-19 infection: a prospective multicenter study. Am J Rhinol Allergy 34(5):686–693. 10.1177/1945892420930954. Epub 2020 Jun 12. (PMID: 32527141; PMCID: PMC7418272) [DOI] [PMC free article] [PubMed]
- 14.Kosugi EM, Lavinsky J, Romano FR, Fornazieri MA, Luz-Matsumoto GR, Lessa MM et al (2020) Incomplete and late recovery of sudden olfactory dysfunction in COVID-19. Braz J Otorhinolaryngol 86(4):490–496. 10.1016/j.bjorl.2020.05.001. Epub 2020 May 25. (PMID: 32534982; PMCID: PMC7247498) [DOI] [PMC free article] [PubMed]
- 15.Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020;58(3):295–298. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 16.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS (2020) Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol 10(7):806–813. 10.1002/alr.22579. Epub 2020 Jun 1. (PMID: 32279441; PMCID: PMC7262089) [DOI] [PMC free article] [PubMed]
- 17.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS (2020) Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol 10(7):821–831. 10.1002/alr.22592. Epub 2020 Jun 7. (PMID: 32329222; PMCID: PMC7264572) [DOI] [PMC free article] [PubMed]
- 19.Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC 3rd (2020) COVID-19 Anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg 163(1):132–134. 10.1177/0194599820922992. Epub 2020 Apr 28. (PMID: 32340555) [DOI] [PubMed]
- 20.Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID-19 pandemic—an observational cohort study. J Otolaryngol Head Neck Surg. 2020;49(1):26. doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechner M, Chandrasekharan D, Jumani K, Liu J, Gane S, Lund VJ, et al. Anosmia as a presenting symptom of SARS-CoV-2 infection in healthcare workers—a systematic review of the literature, case series, and recommendations for clinical assessment and management. Rhinology. 2020;58(4):394–399. doi: 10.4193/Rhin20.189. [DOI] [PubMed] [Google Scholar]
- 22.Hunter E, Price DA, Murphy E, van der Loeff IS, Baker KF, Lendrem D et al (2020) First experience of COVID-19 screening of health-care workers in England. Lancet 395(10234):e77–e78. 10.1016/S0140-6736(20)30970-3. Epub 2020 Apr 22. (PMID: 32333843; PMCID: PMC7176380) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available.



