Table 1. Patients’ demographics and baseline characteristics (phase 1a study and phase1b study) in safety analysis set.
| Characteristics | Phase 1a study | Phase 1b study (375 mg/m2) | ||||
| 250 mg/m2 (n=3) | 375 mg/m2 (n=6) | 500 mg/m2 (n=3) | HLX01 (n=43) | Reference rituximab* (n=43) | ||
| ECOG, Eastern Cooperative Oncology Group; PS, performance status; FL, follicular lymphoma; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; LPHL, Hodgkin’s lymphoma, classic, lymphocyte-predominant; cHL, classical Hodgkin lymphoma; DLBCL, diffuse large B cell lymphoma; MALT, mucosa-associated lymphoid tissue lymphoma; U-BCL, unclassified B cell lymphoma; U-DLBCL/BL, unclassified B cell lymphoma with feature intermediate between diffuse large B-cell lymphoma and Burkitt’s lymphoma; MCL, mantel cell lymphoma; MZL, marginal zone lymphoma; NA, not applicable; *, Reference rituximab was sourced from China (MabThera®; rituximab-CN). | ||||||
| Age [median (range)] (year) | 57.2 (51.6−59.2) | 42.5 (31.5−51.5) | 57.0 (32.1−64.3) | 51.0 (28.6−63.6) | 51.0 (24.2−65.1) | |
| Male [n (%)] | 2 (66.7) | 3 (50.0) | 3 (100) | 21 (48.8) | 21 (48.8) | |
Weight (
 ) (kg)
) (kg)
|
75.0±13.1 | 65.7±19.4 | 78.7±27.1 | 66.2±11.0 | 64.4±10.2 | |
Body surface area (
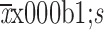 ) (m2)
) (m2)
|
1.8±0.2 | 1.7±0.3 | 1.9±0.3 | 1.7±0.2 | 1.7±0.2 | |
| ECOG PS [n (%)] | ||||||
| 0 | 3 (100) | 5 (83.3) | 3 (100) | 28 (65.1) | 29 (67.4) | |
| 1 | 0 | 1 (16.7) | 0 | 15 (34.9) | 14 (32.6) | |
| Primary tumor type [n (%)] | ||||||
| FL | 0 | 2 (33.3) | 0 | 12 (27.9) | 9 (20.9) | |
| CLL/SLL | 1 (33.3) | 0 | 0 | 2 (4.7) | 2 (4.7) | |
| LPHL | 0 | 1 (16.7) | 0 | 0 | 0 | |
| cHL | 0 | 0 | 1 (33.3) | 0 | 0 | |
| Systemic plasma-cell type CD | 0 | 1 (16.7) | 0 | 0 | 0 | |
| DLBCL | 1 (33.3) | 2 (33.3) | 1 (33.3) | 22 (51.2) | 26 (60.5) | |
| Gastric MALT | 0 | 0 | 1 (33.3) | 0 | 0 | |
| MALT | 1 (33.3) | 0 | 0 | 0 | 0 | |
| U-BCL | 0 | 0 | 0 | 2 (4.7) | 3 (7.0) | |
| U-DLBCL/BL | 0 | 0 | 0 | 0 | 1 (2.3) | |
| MCL | 0 | 0 | 0 | 2 (4.7) | 1 (2.3) | |
| MZL | 0 | 0 | 0 | 3 (7.0) | 1 (2.3) | |
| Tumor stage [n (%)] | ||||||
| I−II | 0 | 4 (66.7) | 1 (33.3) | 20 (46.5) | 13 (30.2) | |
| III−IV | 3 (100) | 2 (33.3) | 2 (66.7) | 23 (53.5) | 30 (69.8) | |
| Duration of disease [median (range)] (year) | NA | NA | NA | 0.9 (0.3−4.3) | 1.3 (0.5−6.5) | |
| Number of chemotherapy regimens [median (range)] | 2.0 (1−4) | 1.5 (0−7) | 2.0 (1−3) | NA | NA | |
| Prior chemotherapy [n (%)] | NA | NA | NA | 34 (79.1) | 34 (79.1) | |
| Prior rituximab [n (%)] | NA | NA | NA | 22 (51.2) | 21 (48.8) | |
