Figure 7: Model of neutrophil-mediated suppression of T cells in the TME.
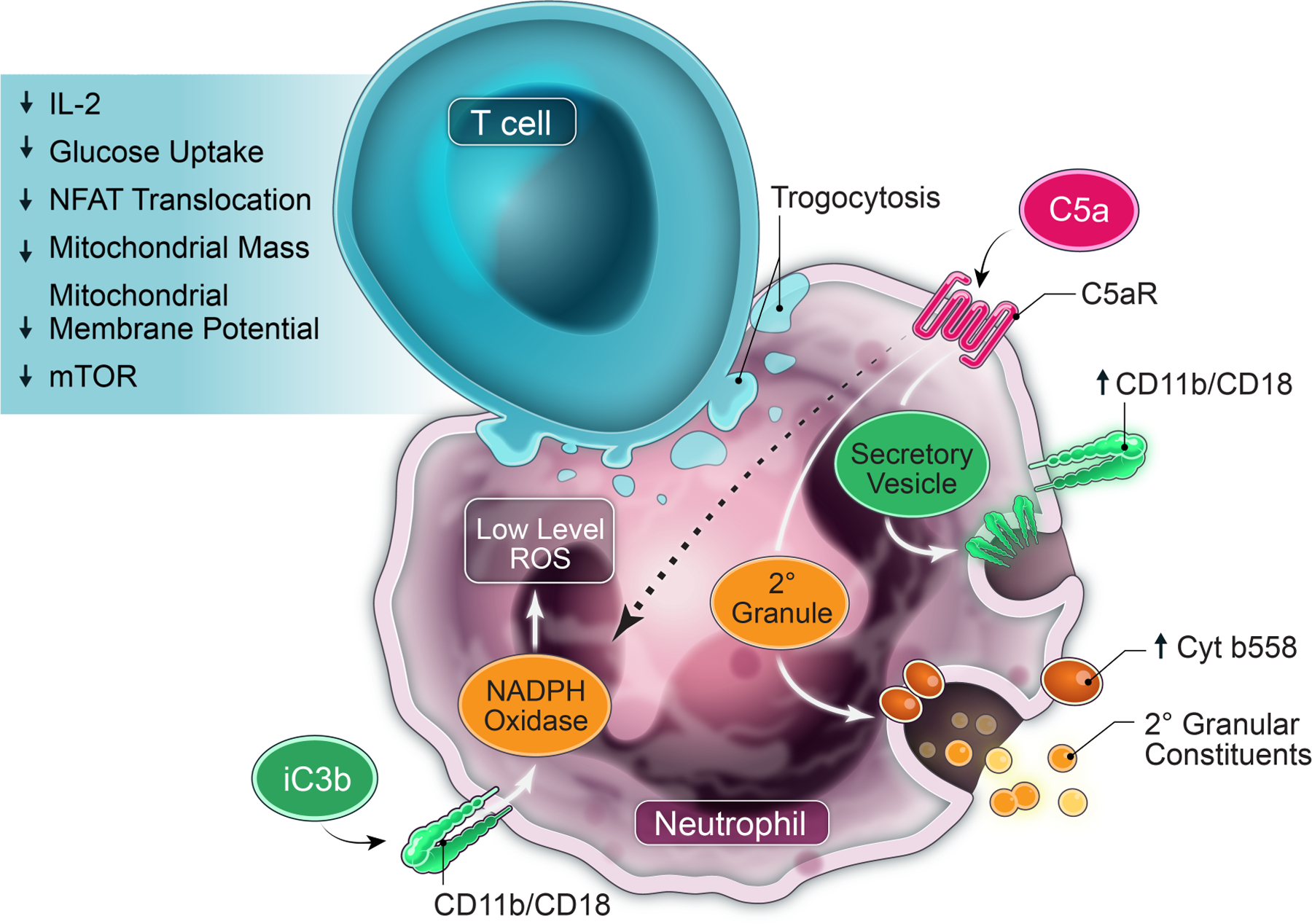
Ovarian cancer ascites fluid and other malignant effusions have multiple products, including DAMPs (25), cytokines, and chemokines (10), that chemoattract and activate neutrophils. Our previously published (8) and current results show that multiple neutrophil effector functions including signaling via CD11b/CD18 (CR3; receptor for iC3b) and C5aR, NADPH oxidase, SNARE-mediated intracellular transport and exocytosis of granular and/or vesicular constituents, protein synthesis, and PS drive this suppressor function. Following exposure of neutrophils to ASC, a positive feedback loop through C5aR activation increases surface expression of CD11b/CD18, and secondary and tertiary granule products, including flavocytochrome b558. ASC-activated neutrophils also increase surface expression of properdin, which stabilizes the alternative pathway C3 convertase to further activate complement. Activated neutrophils cause trogocytosis of T-cell membranes that is CD11b-dependent, likely by enhancing neutrophil adhesion to T cells, though an alternative mechanism could be iC3b binding and signaling. In response to these cumulative signaling and injurious cues, T cells acquire a profound immunoparalysis, characterized by suppression of critical signaling and metabolic pathways necessary for activation and proliferation. These signaling pathways driving neutrophil suppressor function and T-cell non-responsiveness are potential targets for therapeutic modulation.
