FIGURE 2.
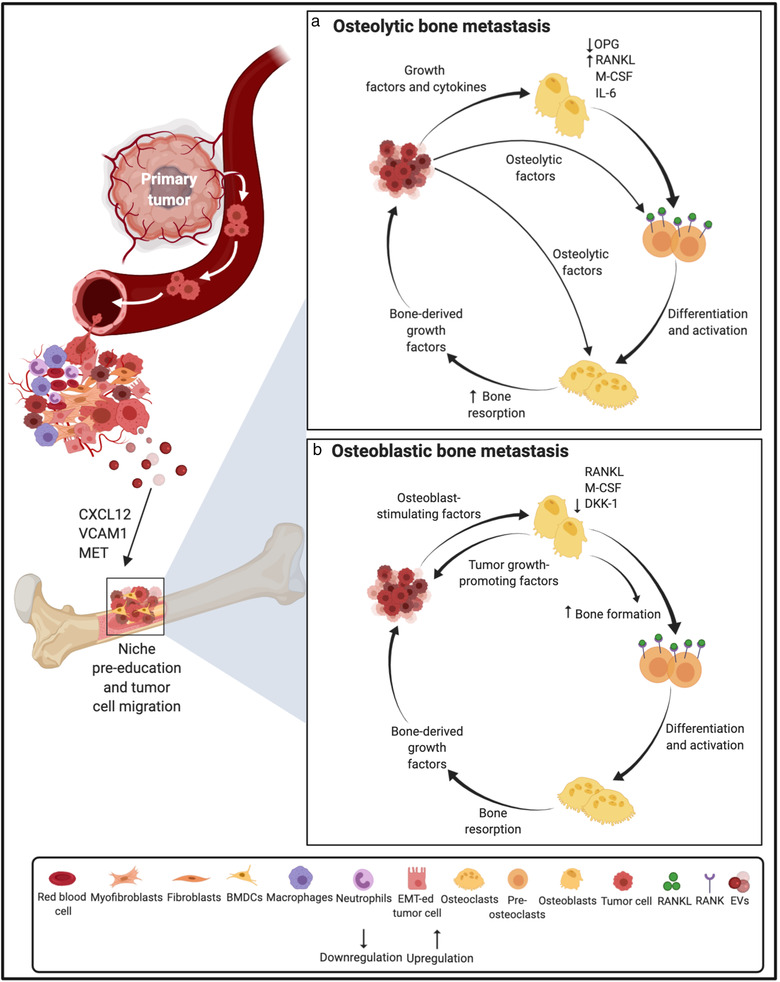
Schematic representation of the role of extracellular vesicles and tumour‐secreted factors in establishing pre‐metastatic niche and osteolytic/osteoblastic metastatic outgrowth in the bone. Primary tumour‐derived molecular components including tumour‐derived secreted factors and EVs play important roles in the modulation of the bone microenvironment, thus promoting metastasis. Prior to the arrival of tumour cells in the bone, EVs can facilitate the establishment of a pre‐metastatic niche in the bone via mechanisms such as the transfer of MET to BMDCs. (a) On establishing a foothold in the bone marrow niche, the osteolytic cancer cells (e.g. from breast cancer) secrete growth factors and cytokines that act on osteoclasts and osteoblasts in the bone microenvironment, such as PTHrP, VEGF, IL‐1, IL‐6, PGE2, TNF‐α, ET‐1 and BMPs. These factors increase the production of M‐CSF and RANKL, an osteoclast differentiating factor, while decreasing OPG (an osteoclastogenesis inhibitory factor) secretion from osteoblasts. The up‐regulated RANKL binds to its receptor RANK on the pre‐osteoclast surface and promotes the maturation of osteoclast precursors into functional osteoclasts and thus, osteolytic activation. Tumour cells also secrete osteolytic factors, most of which act via osteoblast RANKL, that further stimulate osteoclastic bone resorption. Bone resorption causes the release of PDGFs, BMPs, TGF‐β, IGF‐1 and calcium ions that in turn promote cancer cell proliferation, enable continued expression of osteoclast initiating factors and eventually perpetuate a cycle of osteolytic macrometastatic outgrowth. (b) Osteoblastic cancer cells (e.g. from prostate cancer) that have migrated to the bone, adapt to and modify the surrounding microenvironment by secreting osteoblast‐promoting molecules which increase osteoblast differentiation and proliferation including uPA, TGF‐β, VEGF, BMPs, TNF‐α, IGF‐1, Wnt1, WNT3A, ET‐1, PTHrP and adrenomedullin. Activation of the Wnt pathway and the decreased expression of the Wnt antagonist DKK‐1 stimulates osteoblast activity. In turn, enhanced osteoblast activity drives tumour progression by releasing IGF‐1, IL‐6 and IL‐8. Osteoclast activity is also activated in a predominantly osteoblastic lesion through osteoblast‐mediated osteoclastogenesis governed by the increased osteoblastic expression of RANKL and M‐CSF. Accelerated bone matrix degradation promotes the release of growth factors that further enrich the local milieu. Abbreviations: VCAM1, Vascular Cell Adhesion Molecule 1; CXCL12, CXC chemokine ligand 12; PTHrP, Parathyroid hormone‐related protein; PGE2, Prostaglandin E2; ET‐1, Endothelin‐1; BMPs, Bone Morphogenetic Proteins; M‐CSF, Macrophage colony‐stimulating factor; OPG, Osteoprotegerin; PDGF, Platelet‐Derived Growth Factor; IGF‐1, Insulin‐like growth factor 1; uPA, urokinase‐type Plasminogen Activator; and Wnt1, Wingless‐type MMTV integration site 1
